1. Introduction
Chronic kidney disease (CKD) is a major health problem with a considerable worldwide impact and the onset is correlated to the spread of its risk factors such as obesity, metabolic syndrome, arterial hypertension, and diabetes mellitus (DM) [
1,
2].
CKD is directly related with cardiovascular (CV) mortality and morbidity. The latter displayed a 10 to 20-fold increase respect to general population [
3,
4]. This phenomenon cannot only be explained with CV traditional risk factors, but it needs a more accurate assessment that takes into consideration the uremic CV risk factors. Uremic CV risk factors that exert a key role are chronic low-grade inflammatory status [
5], alteration of calcium-phosphorus metabolism [
6,
7,
8,
9], hyperhomocysteinemia [
10], malnutrition [
11], uremic sarcopenia [
12,
13,
14], and oxidative stress (OS) [
15,
16].
The increase in OS is caused by an imbalance between antioxidant defenses and free radical production. Oxygen free radicals are formed in the mitochondria related to aging, DM, CKD, and inflammation [
17]. The increased concentration of pro-oxidant substances influences cellular communication; in the kidney it promotes apoptosis and cell senescence, determining reduced regenerative cellular capacity and fibrosis [
18,
19].
Vitamin C is one of the possible factors linked to the presence of OS in uremic patients [
20,
21]. In fact, it is common to observe a vitamin C deficiency in this population caused by restricted intake of vitamin C-rich foods and by its increased metabolism induced by the chronic low-grade inflammatory status [
22]. Nowadays, several studies are in progress to formulate oral food supplements (OFS) with high ascorbic acid content from natural citrus fruit and rosehip extracts [
23,
24] rather than synthetic ascorbic acid. In this context, it would be very useful to identify natural bioactive compounds, which are able to counteract the abnormal increase of OS and inflammatory status in chronic degenerative non-communicable diseases [
25,
26].
In this study an OFS, enriched in standardized and characterized rosehip natural extract, was tested in both CKD patients and healthy subjects. In particular, the standardized rosehip extract, was chemically characterized for its polyphenols and vitamin C contents.
The aim of this study is to evaluate the effects of Siuper
®, an OFS (based on
Echinacea angustifolia, zinc, rosehip, propolis, and royal jelly) at the dosage of 400 mg per three die, on the inflammatory status, OS, blood toxicity, body composition, and renal function both in CKD patients and in the healthy subjects. For evaluation of inflammatory status we examined the C-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR) [
27].
Furthermore, two biomarkers of blood toxicity and OS such as the erythrocyte glutathione transferase (e-GST) and human oxidized serum albumin (HSAox), respectively, were also monitored in the present study [
19,
28]. e-GST was selected due to its remarkable sensitivity in detecting large spectrum of blood toxins [
12] in a temporal span of a few weeks. Conversely, HSAox is a short-term biomarker of OS and its blood concentration may change in the temporal span of a few hours [
15].
3. Discussion
CKD patients are characterized by an on-going chronic inflammatory state, enhanced OS, and impaired immune response [
33]. These factors are interconnected with an increased CV risk. The identification of a natural OFS, not synthetically produced, standardized in polyphenol and vitamin C contents, which could counteract both inflammation, OS, and modulate the immune response, should be useful. OFS would be a valid tool to improve the quality of life and reduce the CV comorbidity in nephropathic patients on conservative therapy. In our study, we highlighted that the assumption of OFS Siuper
® for 5 weeks significantly decreases traditional inflammatory biomarkers such as CRP, ESR, and a new biomarker of inflammatory status such as PLR. The latter has been correlated to the proinflammatory and prothrombotic status [
34]. Moreover, previous studies have demonstrated that enhanced CRP levels, the acute phase response protein, are linked to higher hospitalization rate and lower serum albumin levels in pre-dialysis patients [
35]. High CRP values are related to an increased carotid-intima media area in pre-dialysis patients, highlighting a direct correlation between the concentration of CRP and oxidized LDL [
36]. Therefore, a possible relationship between the increase in OS and inflammation, in uremic patients, appears evident. However, in this study a significant reduction in terms of OS, monitored by HSAox, was not observed. This result could be interpreted with a limited time of administration of the OFS or with the need to increase the content of polyphenols and vitamin C for each OFS dose.
The OFS crucial ingredients with potential therapeutic impact are rosehip, zinc, and
Echinacea angustifolia. In particular, previous studies showed that zinc levels inversely correlate with pro-inflammatory cytokines concentration, such as IL-6, IL-8, and tumor necrosis factor-α (TNF-α) [
37,
38], regulating their gene expression [
39]. In our OFS the dose of zinc for each capsule is 4.5 mg; therefore, the daily zinc dose taken was 13.5 mg, following with the maximum tolerable daily intake (25 mg/day), as suggested by Italian LARN [
40].
Therefore, the reduction of the inflammatory biomarkers, observed after 5 weeks of OFS treatment, can be partly due to the zinc anti-inflammatory action. Moreover, also
Echinacea angustifolia showed anti-inflammatory properties as reported in an animal study by Aarland et al. [
41]. Thus confirming previous data showing a reduction in inflammatory cell infiltration, in an in vitro study [
42].
Finally, rosehip, rich in vitamin C and polyphenolic compounds, has an antioxidant and anti-inflammatory effect [
43]. An interesting study has demonstrated its anti-inflammatory action on polymorphonuclear chemotaxis, highlighting that this effect is in a “dose-dependent” manner [
44]. An additional effect showed by an aqueous extract from rosehip was the inhibition, in vitro, of COX-1 and COX-2 [
45].
In this study, a significant reduction of e-GST activity, after 5 weeks of OFS supplementation, was observed in CKD patients. The activity of this enzyme can be related to the presence of uremic toxins, as previously demonstrated in uremic patients [
46,
47]. In our study, the reduction of e-GST after OFS treatment in CKD patients (
Table 7) and the lack of HSAox variation (
Figure 1 and
Table 7) indicate that Siuper
® mainly has a detoxifying action. In fact, additional parameters confirm the benefit of OFS in CKD patients through the significant reduction of azotaemia and albuminuria at T1. Decreased azotaemia can be viewed as an expression of an improvement in the state of blood toxicity, confirming the possible detoxifying action of the OFS; whilst albuminuria is a useful indicator of the progression of CKD and is directly correlated with CV risk [
48].
This detoxifying action of OFS would seem to be caused by its content in polyphenols and vitamin C, as both are able to reduce the formation of reactive oxygen species (ROS), playing a key role in the cell’s detoxification [
30,
49]. Moreover, a study in healthy young adults showed that a low serum concentration of ascorbic acid is correlated with the concentration of glutathione, thiols, and total antioxidant capacity [
50].
We also observed an improvement in the lipid profile with a statistically significant reduction in blood triglyceride values at the end of the OFS treatment (
Table 2). This result confirms the possible beneficial activity of the OFS in reducing CV risk [
51]. High blood levels of triglycerides predispose to a higher risk of CV events, especially if associated with other risk factors such as high LDL cholesterol, obesity, and DM [
52,
53].
This OFS effect could be induced by rosehip as evidenced by preceding studies. In fact, in mice the administration of extracts from rosehip at dose 100/200 mg/kg/day caused, after 14 days, a reduction of plasma triglyceride and free fatty acid levels. This action would seem to be exercised by its polyphenolic fraction, in particular
trans-tiliroside, which would seem to promote lipid metabolism [
54].
Another study confirms the lipid-lowering action of rosehip, in fact in the rats the concentration of cholesterol and triglyceride were significantly lower after a rosehip oil diet compared to the healthy subjects [
55].
A statistically significant reduction in transferrin and an increase in ferritin were also shown, while remaining within the normal range (
Table 2). Their synthesis is self-regulated in relation to the martial state, but their concentration can be influenced by other factors unrelated to the iron levels, such as phlogistic status, hepatopathies [
56], malnutrition, and nephrotic syndrome [
57].
With regards to the possible effects of OFS on body composition in CKD patients, no statistically significant changes were observed. In contrast, in healthy subjects we observed an improvement in body composition. Specifically, a reduction in FM and an increase in phase angle, TBW, FFM, MM, and BCMI (
Table 9). Therefore, we can hypothesize that OFS can act at the metabolic level by stimulating energy metabolism [
12]. In CKD patients, this improvement is not observed (
Table 4) supposedly since this condition induces, especially in the final stages, a state of metabolic acidosis, which is responsible for stimulating the ubiquitin-proteasome pathway, which can induce muscle proteolysis [
58,
59]. Therefore, in order to assess the possible beneficial effect of OFS on body composition, it will be necessary to conduct a randomized clinical trial in which the metabolic acidosis will be also evaluated. Moreover, the body composition will be determined not only through bioelectrical impedance analysis (BIA) but also through further diagnostic methods, such as dual-energy X-ray absorptiometry.
In order to demonstrate the correlation between OS decrease and the administration of natural bioactive compounds (such as polyphenols and vitamin C) in CKD, further studies are in progress including the evaluation of plasma OS, total free radicals and total plasma antioxidant capacity in addition to the parameters described in this study.
In conclusion, this OFS would seem useful in the treatment of chronic inflammatory status and blood toxicity in CKD patients. The promising results obtained in our pilot study laid the foundation for a further randomized clinical trial, conducted on a larger number of patients that will be needed to confirm these results.
4. Materials and Methods
4.1. Oral Food Supplement, Analysis of Vitamin C, Polyphenol Total Content and Antioxidant Capacity In Vitro
The OFS was the Siuper® Marispharma, Frosinone, Italy, based on Echinacea angustifolia, zinc, rosehip, propolis, and royal jelly. OFS was administered at the dose of 400 mg per three die, for the evaluation of its possible effects on the inflammatory status, OS, blood toxicity, body composition, and renal function in CKD patients and in the healthy subjects, in our pilot study. The analyzed rosehip dry extract used as an ingredient was supplied by the company Marispharma.
For the extraction of vitamin C, 400 mg of OFS powder or rosehip dry extract were stirred in 4.0 mL 70:30 EtOH:H2O (pH 2.4 by addition of HCOOH) at room temperature for 30 min, centrifuged at 14,000 rpm for 5 min and analyzed. For the extraction of polyphenols, 500 mg of OFS powder or rosehip dry extract were stirred in 25.0 mL of 70:30 EtOH:H2O (pH 3.2 by addition of HCOOH) for 24 h, centrifuged at 14,000 rpm for 5 min and analyzed.
The HPLC-DAD analyses were performed with a liquid chromatography HP-1100 equipped with a DAD detector (Agilent-Technologies, Santa Clara, CA, USA) and a Zorbax SB-aq C18 (150 × 4.6 mm i.d. 5 μm) (Agilent-Technologies, Santa Clara, CA, USA) column. For ascorbic acid a 0.4 mL/min flow was applied for 8 min with a mobile phase 95% H2O (pH 3.2 by addition of HCOOH) (A) 5% CH3CN (B). For the analysis of polyphenols, a multistep linear gradient was applied from 100% A to 100% B in 43 min with a 0.4 mL/min flow.
The qualitative analysis was performed according to chromatographic and spectrophotometric data, by comparison with the specific standards available. The quantification of vitamin C was performed by using a 5-points calibration curve (r2 ≥ 0.999) in ascorbic acid at 260 nm.
Total phenols and polyphenols were evaluated by spectrophotometric Folin-Ciocalteu assay, measuring absorbance at 725 nm of a sample solution containing Folin-Ciocalteu reagent, 20% Na
2CO
3 after 40 min incubation. The calibration curve was obtained using five gallic acid solutions at different concentrations. The phenols content of each sample is expressed as GAEs and correlated with the in vitro antioxidant activity [
30,
31].
All analyses were carried out in triplicate; the results are given as means and the standard error was <3%.
4.2. Chemicals and Reagents
Cystamine, 1-chloro-2,4-dinitrobenzene (CDNB), ethylenediaminetetraacetic acid (EDTA), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) (Ellman’s reagent), glutathione (GSH), formic acid (HCOOH) HPLC grade, acetonitrile (CH3CN) HPLC grade, ethanol (EtOH) HPLC grade, ascorbic acid, Folin–Ciocalteu reactive, sodium carbonate (Na2CO3), and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4.3. Patients
Men and women aged 18–80 years were considered suitable for the study. The study protocol complied with the declaration of Helsinki and was appointed by the Ethical Committee of Fondazione Policlinico Tor Vergata (PTV) of Rome.
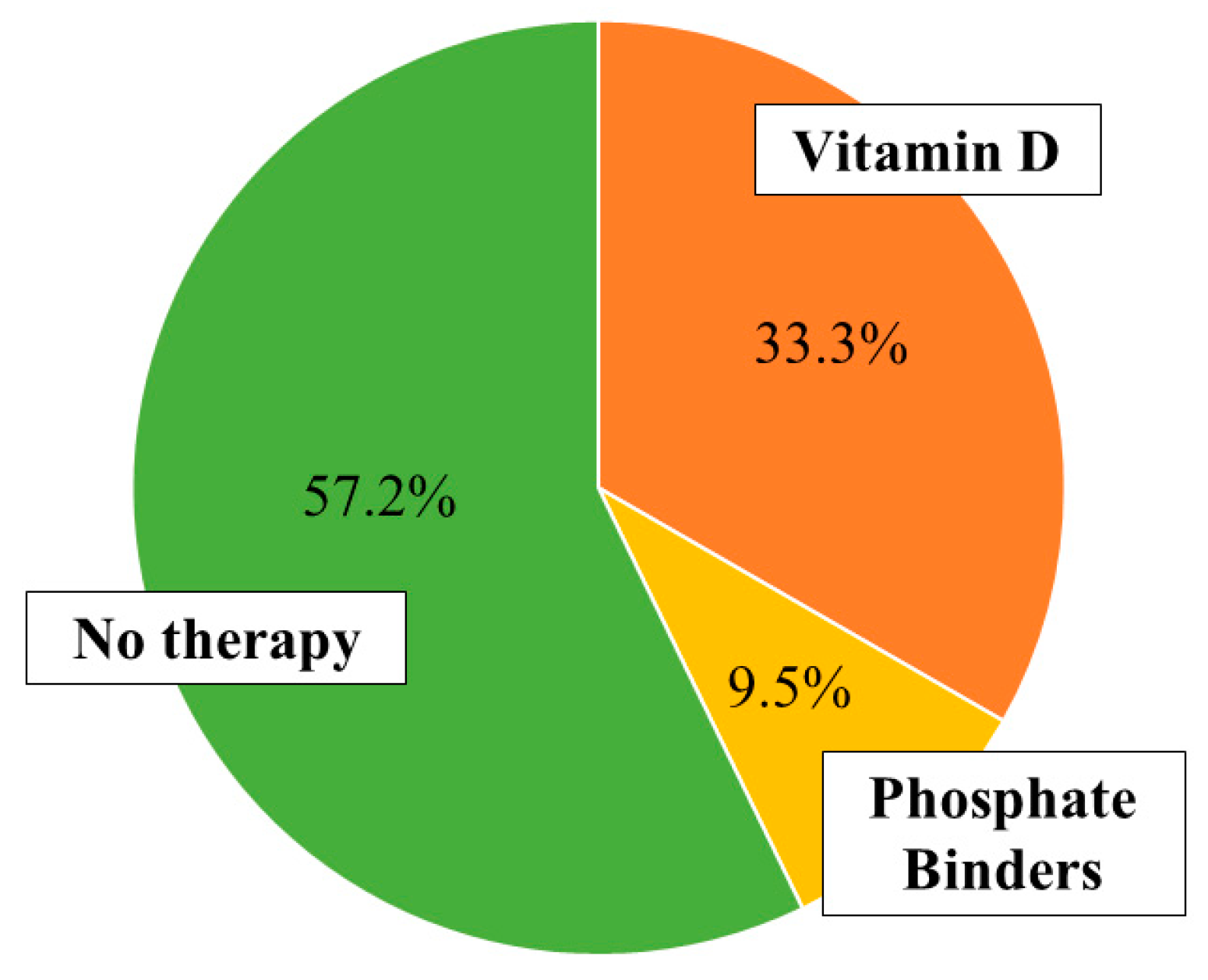
A written fully informed consent was provided to all CKD patients and healthy subjects before enrolment into the study. Exclusion criteria were cancer, virus hepatitis B and C, rheumatologic disorders (e.g., systemic lupus erythematosus), pregnancy, chronic maintenance hemodialysis, body mass index (BMI) <18.5 kg/m2, vitamin D concentration <30 ng/mL, and oral supplements in the last 3 months.
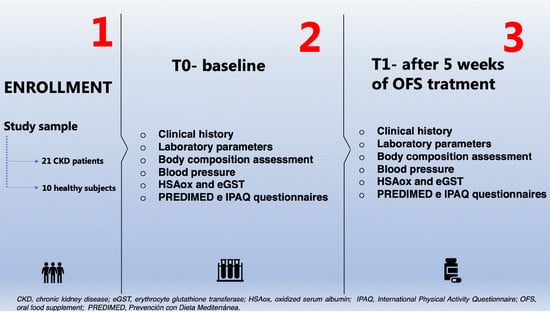
A total of 21 patients (mean age 68.7 ± 10 years), affected by CKD (stage I–IV according to the National Kidney Foundation Kidney—Disease Outcomes Quality Initiative guidelines [
60]), were recruited from Centre of Hypertension and Nephrology Unit of Fondazione PTV, Rome.
In CKD patients, the primary causes of renal failure were glomerulonephritis (10%), nephroangiosclerosis (48%), diabetic nephropathy (14%), chronic pyelonephritis (5%), autosomal dominant polycystic kidney disease (9%), and other causes (14%).
Participation in the study included a complete medical history to gather information about health status, current medications, eating habits, alcohol drinking, smoking, and family history for chronic diseases.
Ten healthy volunteers matched for age, sex, and body composition parameters such as weight, height, and BMI, constituted the healthy subjects.
All enrolled subjects (CKD patients and healthy subjects) were treated for 5 weeks with OFS (400 mg per three die).
A complete evaluation of laboratory parameters and body composition were conducted at two different times of the OFS treatment, at baseline (T0) and after 5 weeks (T1). Only HSAox was monitored every week.
4.4. Anthropometric Measurements and Body Composition Assessment
Anthropometric parameters of all the participants were recorded according to standard methods [
61]. Body weight (kg) was measured to the nearest 0.01 kg, using a balance scale (Seca 711, Hamburg, Germany). Height (m) was measured using a stadiometer to the nearest 0.1 cm (Seca 220, Hamburg, Germany). BMI was calculated as body weight divided by height squared (kg/m
2).
For evaluation of body composition, all enrolled subjects performed BIA. Resistance (R), reactance (Xc), and phase angle at 50 KHz frequency were measured using a BIA 101S instruments (Akern/RIL System-Florence) [
62].
4.5. Laboratory Parameters
For measurement of laboratory parameters, we used an automated hematology analyzer XE-2100 (Sysmex, Kobe, Japan) for the determination of hemoglobin (Hb). All routine parameters were determined using Dimension VISTA 1500 (Siemens Healthcare Diagnostics, Milano, Italy).
The lipid profile, like total-cholesterol (TC), triglyceride, low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL), was determined by standard enzymatic colorimetric techniques (Roche modular P800, Roche diagnostics, Indianapolis, IN, USA).
4.6. Questionnaires
Two questionnaires were administered to the enrolled subjects: Prevención con Dieta Mediterránea (PREDIMED) [
63] and International Physical Activity Questionnaire (IPAQ) [
64] at the beginning and at the end of the study.
The first was administered to assess the adherence to Mediterranean diet, the second was administered to evaluate the degree of physical activity before and after OFS treatment. Both questionnaires were administered in order to avoid possible biases related to eating habits or physical activity on examined parameters in the study.
4.7. Erythrocyte Glutathione Transferase Activity
e-GST activity was assessed using a spectrophotometric assay at 340 nm (37 °C), with an Uvikon 941 Plus spectrophotometer (Kontron Instruments, Watford, Herts, UK). Shortly, 40 μL of whole blood was diluted into 1 mL of bi-distilled water causing immediate erythrocyte hemolysis. After that, 100 µL of hemolyzed blood was diluted to a final volume of 1 mL containing 1 mM GSH, 1 mM CDNB in 0.1 M potassium phosphate buffer pH 6.5 [
65]. Data was reported as enzyme units (U) per gram of hemoglobin (Hb) (U/gHb): one unit is the amount of enzyme that catalyzes the conjugation of 1 micromole of GSH to CDNB in 1 min at 37 °C [
66].
4.8. Human Oxidized Serum Albumin
HSAox was determined by subtracting reduced HSA values from the total HSA. Reduced HSA was determined by exploiting the fast reaction of cystamine with Cys34, the only free cysteine in albumin that is partially present as mixed disulfide (generally with cysteine) depending on the redox status of the human serum. The released cysteamine is stoichiometric with Cys34 and it can be determined with DTNB (ε
412nm = 14,100 M
−1 cm
−1). The assay was performed with a Kontron Uvikon 941 Plus spectrophotometer (Kontron Instruments) at 412 nm at 25 °C. A human serum volume of 50 μL was diluted in 890 μL of potassium phosphate buffer 0.1 M pH 8.0, recording an autozero sample. Then, 50 μL of DTNB (50 μM final concentration) and 10 μL of cystamine (1 mM final concentration) were added to the solution. After an incubation of 15 min at room temperature, the absorbance was recorded [
46]. Total HSA was determined according to the standard laboratory protocols.
4.9. Statistical and Graphical Analysis
Data is reported as mean ± standard deviation for parametric variables and as median (range minimum–maximum) for non-parametric variables. All continuous variables were checked for normality using Kolmogorov–Smirnov test. Differences between the baseline and the final outcomes for parametric values were tested with a paired
t-test. The non-parametric variables data was analyzed for significance with the Wilcoxon test. The minimal level of significance of the differences was fixed at
p < 0.05. Comparison among groups was performed with the univariate ANOVA with a covariate for continuous parametric variables. In our study, every enrolled subject was the control of himself. Furthermore, the short matrices of data of PREDIMED and IPAQ were analyzed with McNemar’s test [
67]. This analysis was performed using the Statistical Package for the Social Sciences Windows, version 15.0 (SPSS, Chicago, Illinois, USA). The graphic and results visualization were obtained by GraphPad Prism (La Jolla, CA, USA).