Safety of Repeated Administration of Parenteral Ketamine for Depression
Abstract
:1. Introduction
2. Results
2.1. Survey
2.2. Literature Review
3. Discussion
4. Methods
4.1. Survey
4.2. Literature Review
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donisi, V.; Tedeschi, F.; Salazzari, D.; Amaddeo, F. Pre- and post-discharge factors influencing early readmission to acute psychiatric wards: Implications for quality-of-care indicators in psychiatry. Gen. Hosp. Psychiatry 2016, 39, 53–58. [Google Scholar] [CrossRef]
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Palazidou, E. The neurobiology of depression. Br. Med. Bull. 2012, 101, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Ketalar [Package Insert] Chestnut Rige, NY. PAR Pharmaceuticals. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/016812s043lbl.pdf (accessed on 1 June 2020).
- Fond, G.; Loundou, A.; Rabu, C.; Macgregor, A.; Lancon, C.; Brittner, M.; Micoulaud-Franchi, J.A.; Richieri, R.; Courtet, P.; Abbar, M.; et al. Ketamine administration in depressive disorders: A systematic review and meta-analysis. Psychopharmacology 2014, 231, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Spravato (esketamine) Nasal Spray Precribing Information. Janssen Pharmaceuticals, Inc.: Titusville, NJ, USA, 2019. Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPRAVATO.-pi.pdf (accessed on 13 November 2019).
- Nemeroff, C.B. Ketamine: Quo Vadis? Am. J. Psychiatry 2018, 175, 297–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lin, D.; Wu, B.; Zhou, W. Ketamine abuse potential and use disorder. Brain Res. Bull. 2016, 126, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahani, R.; Streutker, C.; Dickson, B.; Stewart, R.J. Ketamine-associated ulcerative cystitis: A new clinical entity. Urology 2007, 69, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Lahti, A.C.; Weiler, M.A.; Tamara Michaelidis, B.A.; Parwani, A.; Tamminga, C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 2001, 25, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.J.; Curran, H.V.; Independent Scientific Committee on Drugs (ISCD). Ketamine use: A review. Addiction 2012, 107, 27–38. [Google Scholar] [CrossRef]
- Kalsi, S.S.; Wood, D.M.; Dargan, P.I. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg. Health Threats J. 2011, 4, 7107. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Fong, J.; Galvez, V.; Shelker, W.; Loo, C.K. Side-effects associated with ketamine use in depression: A systematic review. Lancet Psychiatry 2018, 5, 65–78. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Toprak, M.; Turner, M.S.; Levine, S.P.; Katz, R.B.; Sanacora, G. A Survey of the Clinical, Off-Label Use of Ketamine as a Treatment for Psychiatric Disorders. Am. J. Psychiatry 2017, 174, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Anaya, A.M.C.; Choi, J.K.; Lee, C.S.; Oh, E.; Kim, Y.; Moon, J.Y.; Lee, P.B.; Kim, Y.C. Ketamine infusion therapy for chronic pain management in South Korea: A national survey for pain physicians with a narrative review. Medicine 2018, 97, e11709. [Google Scholar] [CrossRef] [PubMed]
- Buckland, D.M.; Crowe, R.P.; Cash, R.E.; Gondek, S.; Maluso, P.; Sirajuddin, S.; Smith, E.R.; Dangerfield, P.; Shapiro, G.; Wanka, C.; et al. Ketamine in the Prehospital Environment: A National Survey of Paramedics in the United States. Prehosp. Disaster Med. 2018, 33, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.M.; Umoren, R.A.; Harris, S.; Strandjord, T.P.; Sawyer, T. Use and perceived safety of stylets for neonatal endotracheal intubation: A national survey. J. Perinatol. 2018, 38, 1331–1336. [Google Scholar] [CrossRef]
- aan het Rot, M.; Collins, K.A.; Murrough, J.W.; Perez, A.M.; Reich, D.L.; Charney, D.S.; Mathew, S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 2010, 67, 139–145. [Google Scholar] [CrossRef]
- Murrough, J.W.; Perez, A.M.; Pillemer, S.; Stern, J.; Parides, M.K.; aan het Rot, M.; Collins, K.A.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 2013, 74, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, W.; Liu, W.; Wang, C.; Zhan, Y.; Li, H.; Chen, L.; Li, M.; Ning, Y. Neurocognitive effects of six ketamine infusions and the association with antidepressant response in patients with unipolar and bipolar depression. J. Psychopharmacol. 2018, 32, 1118–1126. [Google Scholar] [CrossRef]
- Archer, S.; Chrenek, C.; Swainson, J. Maintenance Ketamine Therapy for Treatment-Resistant Depression. J. Clin. Psychopharmacol. 2018, 38, 380–384. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Katz, R.B.; Toprak, M.; Webler, R.; Ostroff, R.B.; Sanacora, G. Acute and Longer-Term Outcomes Using Ketamine as a Clinical Treatment at the Yale Psychiatric Hospital. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; Owoeye, O.; Batten, L.A.; Blier, P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am. J. Psychiatry 2019, 176, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Vande Voort, J.L.; Morgan, R.J.; Kung, S.; Rasmussen, K.G.; Rico, J.; Palmer, B.A.; Schak, K.M.; Tye, S.J.; Ritter, M.J.; Frye, M.A.; et al. Continuation phase intravenous ketamine in adults with treatment-resistant depression. J. Affect. Disord. 2016, 206, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.F.; Swee, M.B.; Pavone, K.J.; Taylor, N.; Akeju, O.; Baer, L.; Nyer, M.; Cassano, P.; Mischoulon, D.; Alpert, J.E.; et al. Rapid and Sustained Reductions in Current Suicidal Ideation Following Repeated Doses of Intravenous Ketamine: Secondary Analysis of an Open-Label Study. J. Clin. Psychiatry 2016, 77, e719–e725. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Bentley, K.H.; Eikermann, M.; Taylor, N.; Akeju, O.; Swee, M.B.; Pavone, K.J.; Petrie, S.R.; Dording, C.; Mischoulon, D.; et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. J. Affect. Disord. 2019, 243, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.B.; Fedgchin, M.; Daly, E.J.; De Boer, P.; Cooper, K.; Lim, P.; Pinter, C.; Murrough, J.W.; Sanacora, G.; Shelton, R.C.; et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am. J. Psychiatry 2016, 173, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, P.R.; Johns, B.; Kuskowski, M.; Wels, J.; Thuras, P.; Albott, C.S.; Lim, K.O. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J. Affect. Disord. 2014, 155, 123–129. [Google Scholar] [CrossRef]
- Diamond, P.R.; Farmery, A.D.; Atkinson, S.; Haldar, J.; Williams, N.; Cowen, P.J.; Geddes, J.R.; McShane, R. Ketamine infusions for treatment resistant depression: A series of 28 patients treated weekly or twice weekly in an ECT clinic. J. Psychopharmacol. 2014, 28, 536–544. [Google Scholar] [CrossRef]
- Bryant, K.A.; Altinay, M.; Finnegan, N.; Cromer, K.; Dale, R.M. Effects of Repeated Intravenous Ketamine in Treatment-Resistant Geriatric Depression: A Case Series. J. Clin. Psychopharmacol. 2019, 39, 158–161. [Google Scholar] [CrossRef]
- George, D.; Galvez, V.; Martin, D.; Kumar, D.; Leyden, J.; Hadzi-Pavlovic, D.; Harper, S.; Brodaty, H.; Glue, P.; Taylor, R.; et al. Pilot Randomized Controlled Trial of Titrated Subcutaneous Ketamine in Older Patients with Treatment-Resistant Depression. Am. J. Geriatr. Psychiatry 2017, 25, 1199–1209. [Google Scholar] [CrossRef]
- Albott, C.S.; Lim, K.O.; Forbes, M.K.; Erbes, C.; Tye, S.J.; Grabowski, J.G.; Thuras, P.; Batres, Y.C.T.M.; Wels, J.; Shiroma, P.R. Efficacy, Safety, and Durability of Repeated Ketamine Infusions for Comorbid Posttraumatic Stress Disorder and Treatment-Resistant Depression. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef] [PubMed]
- Segmiller, F.; Ruther, T.; Linhardt, A.; Padberg, F.; Berger, M.; Pogarell, O.; Moller, H.J.; Kohler, C.; Schule, C. Repeated S-ketamine infusions in therapy resistant depression: A case series. J. Clin. Pharmacol. 2013, 53, 996–998. [Google Scholar] [CrossRef]
- Szymkowicz, S.M.; Finnegan, N.; Dale, R.M. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J. Affect. Disord. 2013, 147, 416–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blier, P.; Zigman, D.; Blier, J. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol. Psychiatry 2012, 72, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Diaz, A.; Fernandez-Gonzalez, J.L.; Lujan-Jimenez, J.E.; Galiano-Rus, S.; Gutierrez-Rojas, L. Use of repeated intravenous ketamine therapy in treatment-resistant bipolar depression with suicidal behaviour: A case report from Spain. Ther. Adv. Psychopharmacol. 2017, 7, 137–140. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Blasey, C.; Sudheimer, K.; Pannu, J.; Pankow, H.; Hawkins, J.; Birnbaum, J.; Lyons, D.M.; Rodriguez, C.I.; et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 2018, 175, 1205–1215. [Google Scholar] [CrossRef]
- Schak, K.M.; Vande Voort, J.L.; Johnson, E.K.; Kung, S.; Leung, J.G.; Rasmussen, K.G.; Palmer, B.A.; Frye, M.A. Potential Risks of Poorly Monitored Ketamine Use in Depression Treatment. Am. J. Psychiatry 2016, 173, 215–218. [Google Scholar] [CrossRef]
- Riva-Posse, P.; Reiff, C.M.; Edwards, J.A.; Job, G.P.; Galendez, G.C.; Garlow, S.J.; Saah, T.C.; Dunlop, B.W.; McDonald, W.M. Blood pressure safety of subanesthetic ketamine for depression: A report on 684 infusions. J. Affect. Disord. 2018, 236, 291–297. [Google Scholar] [CrossRef]
- KRIYA Ketamine Research Institute. Available online: https://www.kriyainstitute.com/ (accessed on 24 May 2019).
- Ketamine Advocacy Network. Available online: http://www.ketamineadvocacynetwork.org/mission-and-vision/ (accessed on 24 May 2019).
- SurveyMonkey Inc. San Mateo, California, USA. Available online: www.surveymonkey.com (accessed on 24 May 2019).
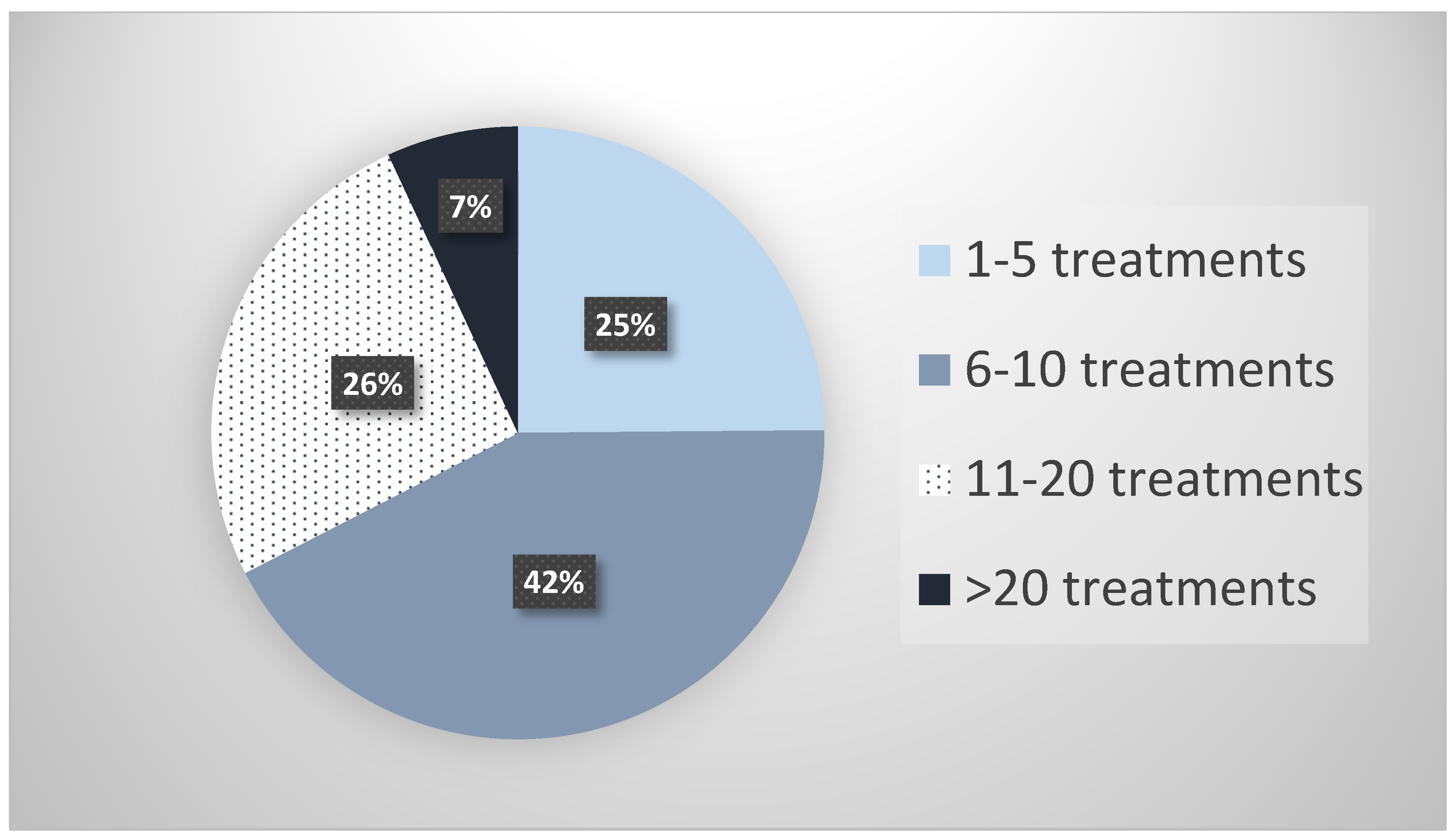
| Specialty | N (%) |
| Psychiatry | 14 (51.9%) |
| Anesthesia | 7 (25.9%) |
| Emergency Medicine | 4 (14.8%) |
| Primary Care | 1 (3.7%) |
| Critical Care | 1 (3.7%) |
| Pain and Palliative Care | 0 (0%) |
| Practice Setting | N (%) |
| Private Practice | 25(92.6%) |
| Academic Medical Center | 0 (0%) |
| Non-Academic Medical Center | 2 (7.4%) |
| Adverse Effect | Cases Requiring Discontinuation * N (%) |
|---|---|
| Bladder Dysfunction | 3 (0.06%) |
| Addiction ** | 0 (NA) |
| Psychotic Symptoms | 1 (2%) |
| Cognitive Deficits | 0 (NA) |
| Psychological Distress | 33 (0.5%) |
| Hypomania | 2 (0.03%) |
| Hypertension | 0 (NA) |
| Nausea *** | 6 (0.09%) |
| Respiratory Distress *** | 1 (0.02%) |
| Seizure *** | 1(0.02%) |
| Total | 47 (0.7%) |
| Report | Number of Patients | Ketamine Dose and Rate | Route | Dis-Continuation Due to AE | Other Notable AEs | Notes |
|---|---|---|---|---|---|---|
| Aan het Rot et al. [19] | 10 | 0.5 mg/kg /40 min | IV | 0 | 0 | No significant AEs |
| Murrough et al. [20] | 24 | 0.5 mg/kg/40 min | IV | 0 | 0 | No significant AEs |
| Zhou et al. [21] | 84 | 0.5 mg/kg/40 min | 1 (1.1%) | 0 | One patient withdrew due to a manic switch | |
| Archer et al. [22] | 11 | 0.5 mg/kg/40 min | IV | 0 | 0 | No significant AEs |
| Wilkinson et al. [23] | 14 | 0.5 mg/kg/40 min | IV | 0 | 0 | No significant AEs |
| Phillips et al. [24] | 41 | 0.5 mg/kg/40 min | IV | 0 | 0 | No serious AE and no evidence of craving or drug-seeking behavior during the infusions or follow-up |
| Vande Voort et al. [25] | 12 | 0.5 mg/kg/40 min | IV | 0 | 2 (1.7%) | 1 subject developed behavioral outbursts and suicide threats during follow-up while hospitalized, and another died by suicide several weeks after the end of follow-up. Authors did not attribute events these AEs to ketamine treatments |
| Ionescu et al. [26] | 14 | 0.5 mg/kg/40 min for first 3 infusions; 75 mg/kg/40 min for last three | IV | Not reported | Not reported | AEs not reported |
| Ionescu et al. [27] | 26 | 0.5 mg/kg/45 min | IV | 0 | 0 | |
| Singh et al. [28] | 21 | 0.5 mg/kg/40 min | IV | 0 | 0 | 15–28% (twice weekly infusions) 6–41% (thrice weekly infusions) All symptoms resolved within 3 h of the infusions |
| Shiroma et al. [29] | 12 | 0.5 mg/kg/40 min | IV | 0 | 2 (1.7%) | Single episode of vomiting effectively countered by IV labetalol and ondansetron |
| Diamond et al. [30] | 28 | 0.5 mg/kg/40 min | IV | 2 (7.1%) | 5 (17.9%) | Two patients discontinued due to adverse reactions |
| Bryant et al. [31] | 6 | 0.5 mg/kg/40 min | IV | 0 | 5 (83.3%) | panic attack (n = 1), rapid cycling of mood with a mild hypomanic episode (n = 1), emesis (n = 2) during treatment, symptomatic cystitis (n = 1), and a hypnogogic hallucination (n = 1) |
| George et al. [32] | 10 | 0.3–0.5 mg/kg | SC | 0 | 2 (20%) | One patient reported the urge to urinate slightly more often. One patient had elevated aspartate aminotransferase (42–60) and alanine aminotransferase (25–44) after 12 treatments of 0.5 mg/kg |
| Albott et al. [33] | 15 | 0.5 mg/kg/40 min | IV | 0 | 0 | No significant AEs |
| Segmiller et al. [34] | 6 | 0.25 mg/kg/40 min | IV | 1 (16.7%) | 1 (16.7%) | One patient decided to discontinue before receiving all infusions due to the transient dissociative effects |
| Szymkowicz et al. [35] | 3 | 0.5 mg/kg/40 min | IV | 0 | 2 (66.7%) | Transient hypomanic states over 12 months |
| Blier et al. [36] | 1 | 0.5 mg/kg/40 min for most infusions | IV | 0 | 0 | No significant AEs |
| Lopez-Diaz et al. [37] | 1 | 0.5 mg/kg/40 min | IV | 0 | 1 (100%) | Transient sedation during infusion |
| TOTAL | 339 | 4 (1.2%) | 20 (5.9%) | Combined 24 (7.1%) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feifel, D.; Dadiomov, D.; C. Lee, K. Safety of Repeated Administration of Parenteral Ketamine for Depression. Pharmaceuticals 2020, 13, 151. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070151
Feifel D, Dadiomov D, C. Lee K. Safety of Repeated Administration of Parenteral Ketamine for Depression. Pharmaceuticals. 2020; 13(7):151. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070151
Chicago/Turabian StyleFeifel, David, David Dadiomov, and Kelly C. Lee. 2020. "Safety of Repeated Administration of Parenteral Ketamine for Depression" Pharmaceuticals 13, no. 7: 151. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070151




