The In Vitro Anti-amyloidogenic Activity of the Mediterranean Red Seaweed Halopithys Incurva
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Procedure and Biochemical Composition of the H. Incurva Extracts
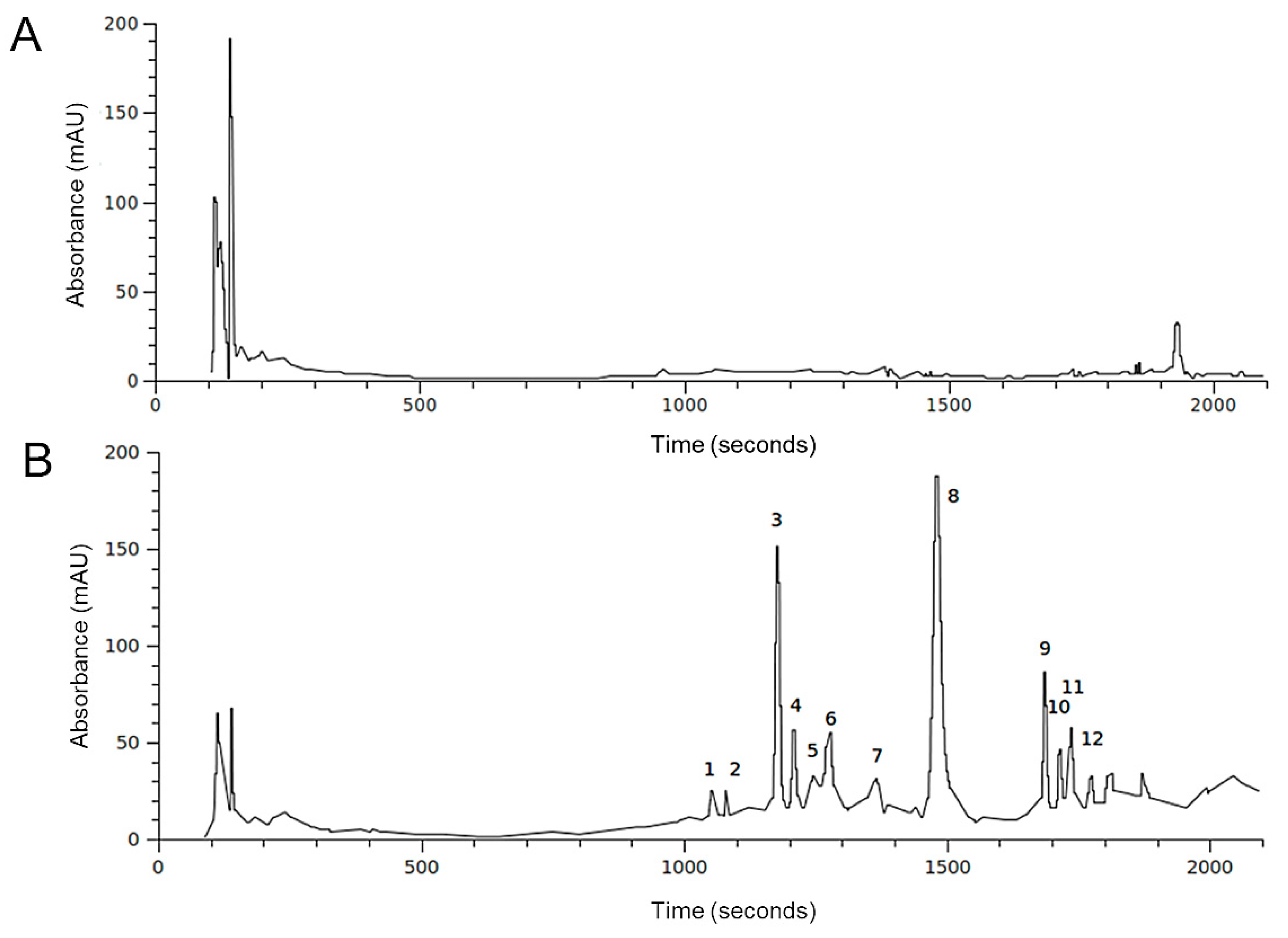
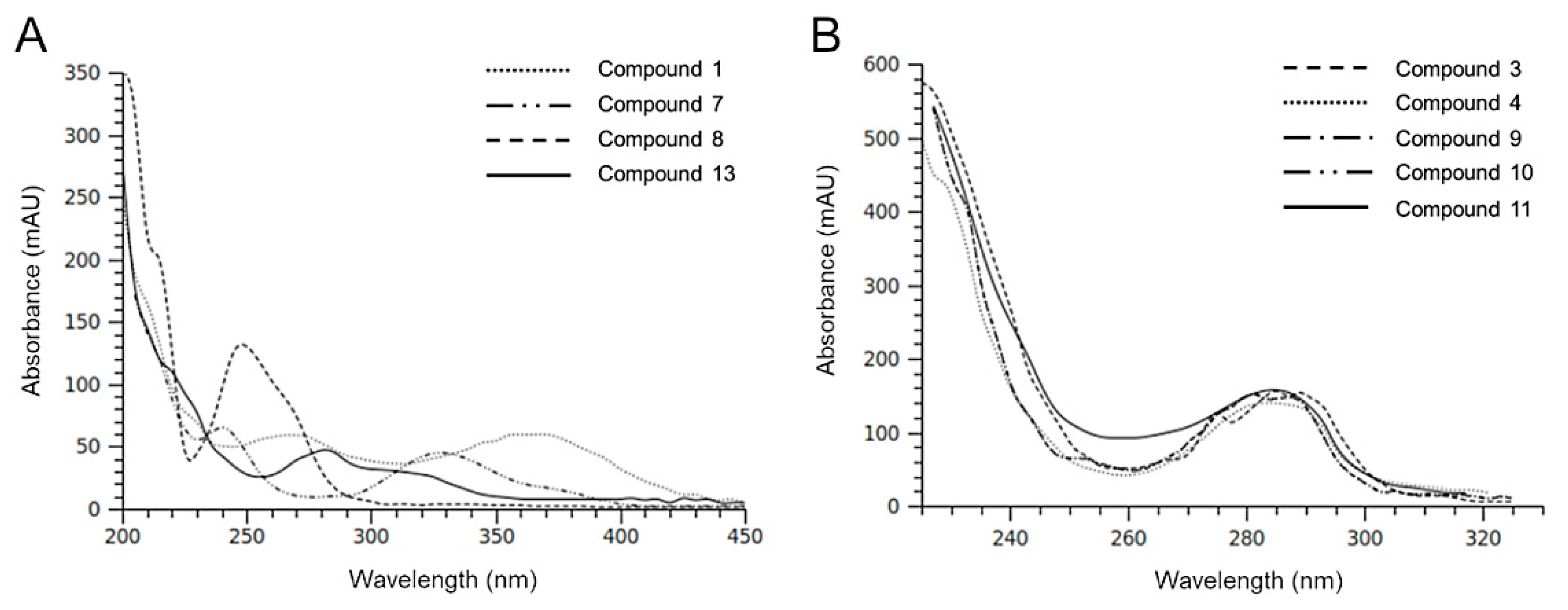
2.2. HPLC-DAD-MS Analysis of H. Incurva Extracts
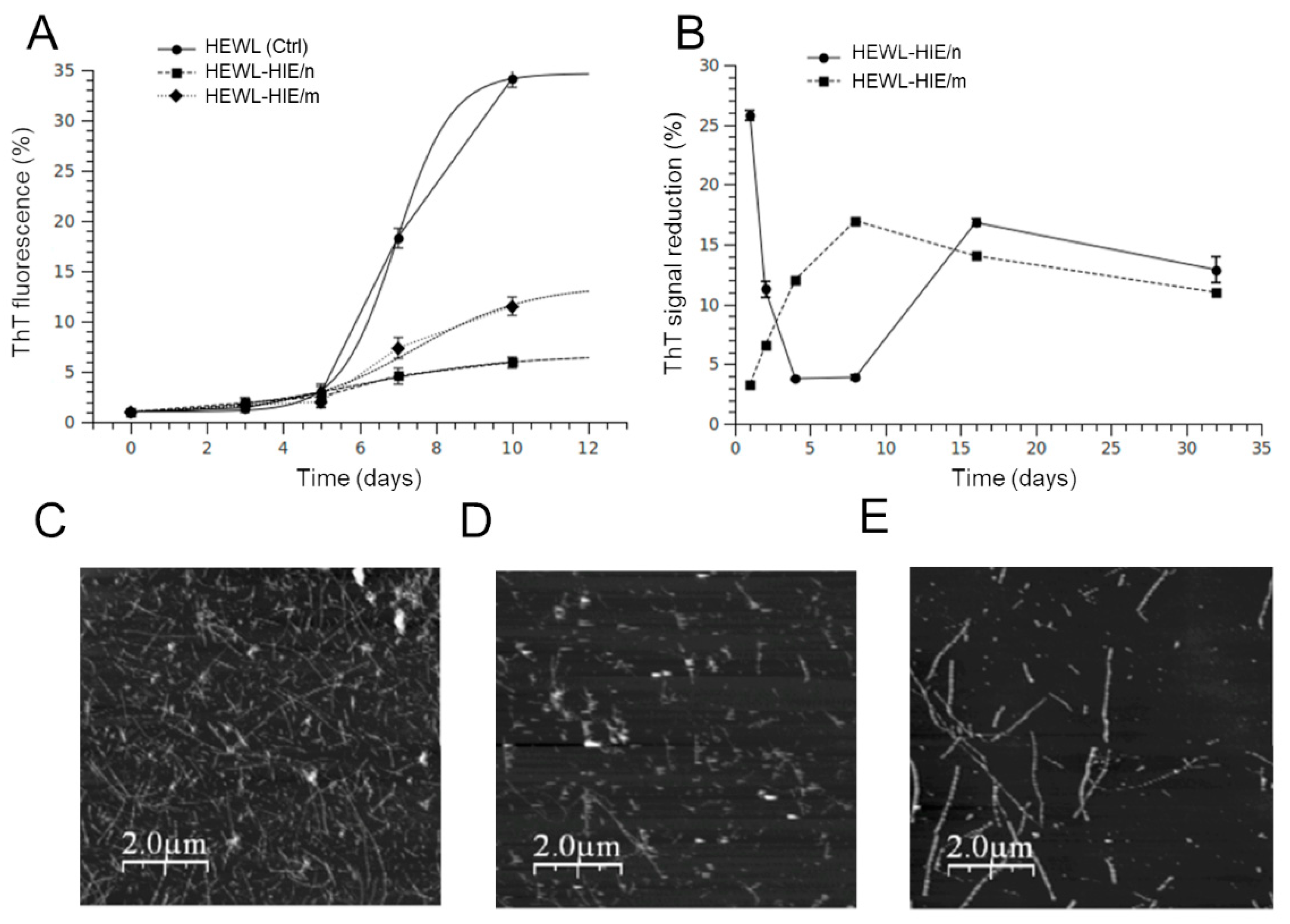
2.3. Effect of H. Incurva Extracts on HEWL Amyloid Fibril Formation
2.4. Evaluation of HEWL Amyloid Aggregate-Induced Cytotoxicyty
3. Materials and Methods
3.1. Materials and Reagents
3.2. H. Incurva Collection and Extraction
3.3. Total Phenolic and Carbohydrate Content
3.4. Antioxidant and Radical Scavenging Assays
3.5. HPLC-DAD-MS Analysis
3.6. Amyloid Fibrils’ Preparation
3.7. Atomic Force Microscopy
3.8. Cell Line
3.9. Cytotoxicity Evaluation
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Noble, W.; Burns, M.P. Challenges in neurodegeneration research. Front. Psychiatry 2010, 1, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currais, A.; Fischer, W.; Maher, P.; Schubert, D. Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain. FASEB J. 2017, 31, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgetti, S.; Greco, C.; Tortora, P.; Aprile, F.A. Targeting Amyloid Aggregation: An Overview of Strategies and Mechanisms. Int. J. Mol. Sci. 2018, 19, 2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, M.; Rigacci, S. Protein folding and aggregation into amyloid: The interference by natural phenolic compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef] [Green Version]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New Drugs from Marine Organisms in Alzheimer’s Disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs. 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Talarico, L.; Maranzana, G. Light and adaptive responses in red macroalgae: An overview. J. Photochem. Photobiol. B 2000, 56, 1–11. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nanteuil, G.; Mastagli, P. A bromophenol in the red alga Halopitys incurvus. Phytochemistry 1981, 20, 1750–1751. [Google Scholar] [CrossRef]
- Chibi, F.; Rchid, H.; Arsalane, W.; Nmila, R. Antioxidant activity and total phenolic content of the red alga Halopitys incurvus harvested from El Jadida Coast (Morocco). Int. J. Pharm. Phytochem. Res. 2018, 10, 176–181. [Google Scholar]
- Oumaskour, K.; Boujaber, N.; Etahiri, S.; Assobhei, O. Anti-inflammatory and antimicrobial activities of twenty-three marine red algae from the coast of sidi bouzid (el jadida-morocco). Int. J. Pharm. Pharm. Sci. 2013, 5, 145–149. [Google Scholar]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Itoe, M.A.; Blunden, G.; Mota, M.M.; Tasdemir, D. Assessment of dual life stage antiplasmodial activity of british seaweeds. Mar. Drugs 2013, 11, 4019–4034. [Google Scholar] [CrossRef] [Green Version]
- Allmendinger, A.; Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother. Res. 2010, 24, 1099–1103. [Google Scholar] [CrossRef]
- Díaz, R.T.A.; Chabrillón, M.; Cabello-Pasini, A.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Characterization of polysaccharides from Hypnea spinella (Gigartinales) and Halopithys incurva (Ceramiales) and their effect on RAW 264.7 macrophage activity. J. Appl. Phycol. 2010, 2, 523–528. [Google Scholar] [CrossRef]
- Zbakh, H.; Salhi, G.; Moussa, H.; Riadi, H. Cytotoxic and antioxidant activities of the red seaweed Halopithys incurva. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 2277–4688. [Google Scholar]
- Ngoungoure, V.L.; Schluesener, J.; Moundipa, P.F.; Schluesener, H. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015, 59, 8–20. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syad, A.N.; Devi, K.P. Assessment of anti-amyloidogenic activity of marine red alga G. acerosa against Alzheimer’s beta-amyloid peptide 25–35. Neurol. Res. 2015, 37, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shariatizi, S.; Meratan, A.A.; Ghasemi, A.; Nemat-Gorgani, M. Inhibition of amyloid fibrillation and cytotoxicity of lysozyme fibrillation products by polyphenols. Int. J. Biol. Macromol. 2015, 80, 95–106. [Google Scholar] [CrossRef]
- Leri, M.; Nosi, D.; Natalello, A.; Porcari, R.; Ramazzotti, M.; Chiti, F.; Bellotti, V.; Doglia, S.M.; Stefani, M.; Bucciantini, M. The polyphenol Oleuropein aglycone hinders the growth of toxic transthyretin amyloid assemblies. J. Nutr. Biochem. 2016, 30, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Mahdavimehr, M.; Meratan, A.A.; Ghobeh, M.; Ghasemi, A.; Saboury, A.A.; Nemat-Gorgani, M. Inhibition of HEWL fibril formation by taxifolin: Mechanism of action. PLoS ONE 2017, 12, e0187841. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Bellotti, V. Lysozyme: A paragmatic molecule for the investigation of protein structure, function and misfolding. Clin. Chim. Acta 2005, 357, 168–172. [Google Scholar] [CrossRef]
- Dumoulin, M.; Kumita, J.R.; Dobson, C.M. Normal and aberrant biological self-assembly: Insights from studies of human lysozyme and its amyloidogenic variants. Acc. Chem. Res. 2006, 39, 603–610. [Google Scholar] [CrossRef]
- Frare, E.; Polverino De Laureto, P.; Zurdo, J.; Dobson, C.M.; Fontana, A. A highly amyloidogenic region of hen lysozyme. J. Mol. Biol. 2004, 340, 1153–1165. [Google Scholar] [CrossRef]
- Ramazzotti, M.; Melani, F.; Marchi, L.; Mulinacci, N.; Gestri, S.; Tiribilli, B.; Degl’Innocenti, D. Mechanisms for the inhibition of amyloid aggregation by small ligands. Biosci. Rep. 2016, 36, e00385. [Google Scholar] [CrossRef] [Green Version]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adh. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajisaka, K.; Agawa, S.; Nagumo, S.; Kurato, K.; Yokoyama, T.; Arai, K.; Miyazaki, T. Evaluation and comparison of the antioxidative potency of various carbohydrates using different methods. J. Agric. Food Chem. 2009, 57, 3102–3107. [Google Scholar] [CrossRef] [PubMed]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Pedersén, M.; Dasilva, E.J. Simple Brominated Phenols in the Bluegreen Alga Calothrix brevissima West. Planta 1973, 115, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Pedersén, M.; Saenger, P.; Fries, L. Simple brominated phenols in red algae. Phytochemistry 1974, 13, 2273–2279. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, X.; Wang, S.; Li, S.; Shang, S.; Yang, Y.; Xu, N.; Lü, Y.; Shi, J. Bromophenol derivatives from the red alga Rhodomela confervoides. J. Nat. Prod. 2004, 67, 1032–1035. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Linke, R.P. A molecular history of the amyloidoses. J. Mol. Biol. 2012, 421, 142–159. [Google Scholar] [CrossRef]
- Velander, P.; Wu, L.; Henderson, F.; Zhang, S.; Bevan, D.R.; Xu, B. Natural product-based amyloid inhibitors. Biochem. Pharmacol. 2017, 139, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Koo, E.H.; Lansbury, P.T., Jr.; Kelly, J.W. Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. USA 1999, 96, 9989–9990. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.E.; Robinson, J.; Matthews, G.; Muschol, M. Amyloid protofibrils of lysozyme nucleate and grow via oligomer fusion. Biophys. J. 2009, 96, 3781–3790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Method Enzymol. 2001, 335, 190–203. [Google Scholar] [CrossRef]
- Ramazzotti, M.; Paoli, P.; Tiribilli, B.; Viglianisi, C.; Menichetti, S.; Degl’Innocenti, D. Catechol-Containing Hydroxylated Biomimetic 4-Thiaflavanes as Inhibitors of Amyloid Aggregation. Biomimetics 2017, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Shanmuganathan, B.; Sheeja Malar, D.; Sathya, S.; Pandima Devi, K. Antiaggregation Potential of Padina gymnospora against the Toxic Alzheimer’s Beta-Amyloid Peptide 25–35 and Cholinesterase Inhibitory Property of Its Bioactive Compounds. PLoS ONE 2015, 10, e0141708. [Google Scholar] [CrossRef] [Green Version]
- Stefani, M. Structural features and cytotoxicity of amyloid oligomers: Implications in Alzheimer’s disease and other diseases with amyloid deposits. Prog. Neurobiol. 2012, 99, 226–245. [Google Scholar] [CrossRef]
- Shaham-Niv, S.; Rehak, P.; Zaguri, D.; Levin, A.; Adler-Abramovich, L.; Vuković, L.; Král, P.; Gazit, E. Differential inhibition of metabolite amyloid formation by generic fibrillation-modifying polyphenols. Comms. Chem. 2018, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Leri, M.; Natalello, A.; Bruzzone, E.; Stefani, M.; Bucciantini, M. Oleuropein aglycone and hy-droxytyrosol interfere differently with toxic Aβ1–42 aggregation. Food Chem. Toxicol. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Palazzi, L.; Bruzzone, E.; Bisello, G.; Leri, M.; Stefani, M.; Bucciantini, M.; Polverino de Laureto, P. Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Sci. Rep. 2018, 8, 8337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Nichino, D.; Relini, A.; Berti, A.; Stefani, M. Aβ(1–42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr. Alzheimer Res. 2011, 8, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Bioactive Compounds from Posidonia oceanica (L.) Delile Impair Malignant Cell Migration through Autophagy Modulation. Mar. Drugs 2018, 16, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Morozova-Roche, L.A.; Zurdo, J.; Spencer, A.; Noppe, W.; Receveur, V.; Archer, D.B.; Joniau, M.; Dobson, C.M. Amyloid fibril formation and seeding by wild-type human lysozyme and its disease-related mutational variants. J. Struct. Biol. 2000, 130, 339–351. [Google Scholar] [CrossRef]
- LeVine, H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: De-tection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]


| HIE/m | HIE/n | |
|---|---|---|
| Starting dry seaweed (g) | 4 | 4 |
| Total extract (mg) | 58 ± 0.4 | 520 ± 41 |
| Extraction yield (%) | 1.45 ± 0.01 | 13 ± 1.02 |
| mg/g respect to dry seaweed | mg/g respect to dry seaweed | |
| Total phenols * | 1 ± 0.01 | 4.85 ± 0.1 |
| Carbohydrates ** | 4.57 ± 0.45 | 10.48 ± 0.45 |
| Antioxidant power *** | 1.99 ± 0.21 | 3.22 ± 0.23 |
| Radical scavenging *** | 0.66 ± 0.05 | 10.73 ± 0.58 |
| mg/g respect to dry extract | mg/g respect to dry extract | |
| Total phenols * | 69.35 ± 1.13 | 37.34 ± 0.79 |
| Carbohydrates ** | 315.34 ± 31.01 | 80.59 ± 3.84 |
| Antioxidant power *** | 137.39 ± 14.70 | 24.84 ±1.82 |
| Radical scavenging *** | 45.67 ± 3.62 | 82.57 ± 0.49 |
| Compound | Negative Ions (m/z) | Br Atoms (n°) |
|---|---|---|
| 3 | 345; 347 [M-H]–; 267; 201; 199; 145; 102 | 1 |
| 4 | 345; 347 [M-H]–; 267; 201; 199; 145; 102 | 1 |
| 5 | 578; 576; 574 [M-H]–; 375; 373; 216; 214 | - |
| 6 | 578; 576; 574 [M-H]–; 373; 375; 216, 214 | - |
| 8 | 217; 215 [M-H]–; 135 | - |
| 9 | 549; 547; 545 [M-H]–; 465; 467; 401; 403; 345; 347; 319; 321; 211 | 2 |
| 10 | 549; 547; 545 [M-H]–; 465; 467; 401; 403; 345; 347; 319; 321; 211 | 2 |
| 11 | 549; 547; 545 [M-H]–; 465; 467; 401; 403; 345; 347; 319; 321; 211 | 2 |
| 12 | 958; 746; 211 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; Ramazzotti, M.; Tiribilli, B.; Barletta, E.; Pretti, C.; Mulinacci, N.; Degl’Innocenti, D. The In Vitro Anti-amyloidogenic Activity of the Mediterranean Red Seaweed Halopithys Incurva. Pharmaceuticals 2020, 13, 185. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13080185
Vasarri M, Ramazzotti M, Tiribilli B, Barletta E, Pretti C, Mulinacci N, Degl’Innocenti D. The In Vitro Anti-amyloidogenic Activity of the Mediterranean Red Seaweed Halopithys Incurva. Pharmaceuticals. 2020; 13(8):185. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13080185
Chicago/Turabian StyleVasarri, Marzia, Matteo Ramazzotti, Bruno Tiribilli, Emanuela Barletta, Carlo Pretti, Nadia Mulinacci, and Donatella Degl’Innocenti. 2020. "The In Vitro Anti-amyloidogenic Activity of the Mediterranean Red Seaweed Halopithys Incurva" Pharmaceuticals 13, no. 8: 185. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13080185







