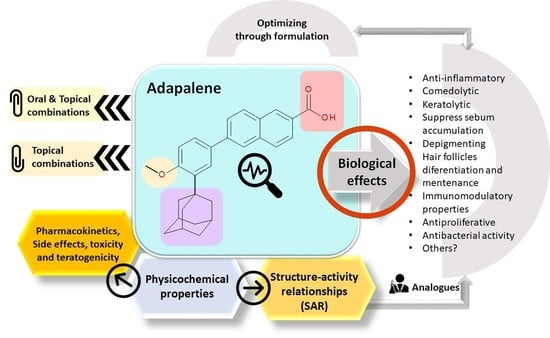
Recent Advances Regarding the Therapeutic Potential of Adapalene
Abstract
:1. Introduction
2. Physicochemical Properties of ADP
3. Mechanism of Action
4. Structure–Activity Relationships (SAR)
5. Biological Effects
5.1. Anti-Inflammatory and Comedolytic Effects
5.2. Keratolytic Effect
5.3. Immunomodulatory Effect
5.4. Antiproliferative Effect
5.5. Neuroprotector Effect
5.6. Antibacterial Activity
5.7. Other Effects
6. Pharmacokinetic Data
7. Side Effects, Toxicity, and Teratogenicity
8. Combinations of ADP with Other APIs
9. Analog of Retinoids
10. The Pharmaceutical Formulation in Optimizing the Biological Properties of ADP
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the Treatment of Skin Aging: An Overview of Clinical Efficacy and Safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Spilovska, K.; Zemek, F.; Korabecny, J.; Nepovimova, E.; Soukup, O.; Windisch, M.; Kuca, K. Adamantane—A Lead Structure for Drugs in Clinical Practice. Curr. Med. Chem. 2016, 23, 3245–3266. [Google Scholar] [CrossRef]
- Waugh, J.; Noble, S.; Scott, L.J. Adapalene: A Review of Its Use in the Treatment of Acne Vulgaris. Drugs 2004, 64, 1465–1478. [Google Scholar] [CrossRef]
- Krautheim, A.; Gollnick, H. Transdermal Penetration of Topical Drugs Used in the Treatment of Acne. Clin. Pharmacokinet. 2003, 42, 1287–1304. [Google Scholar] [CrossRef]
- Kolli, S.S.; Pecone, D.; Pona, A.; Cline, A.; Feldman, S.R. Topical Retinoids in Acne Vulgaris: A Systematic Review. Am. J. Clin. Dermatol. 2019, 20, 345–365. [Google Scholar] [CrossRef]
- Burkhart, C.; Morrell, D.; Goldsmith, L. Dermatological Pharmacology. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postepy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Kassir, M.; Karagaiah, P.; Sonthalia, S.; Katsambas, A.; Galadari, H.; Gupta, M.; Lotti, T.; Wollina, U.; Abdelmaksoud, A.; Grabbe, S.; et al. Selective RAR Agonists for Acne Vulgaris: A Narrative Review. J. Cosmet. Dermatol. 2020, 19, 1278–1283. [Google Scholar] [CrossRef]
- Scott, L.J. Trifarotene: First Approval. Drugs 2019, 79, 1905–1909. [Google Scholar] [CrossRef]
- Millikan, L.E. Adapalene: An Update on Newer Comparative Studies between the Various Retinoids. Int. J. Dermatol. 2000, 39, 784–788. [Google Scholar] [CrossRef]
- Leyden, J.J.; Shalita, A.; Thiboutot, D.; Washenik, K.; Webster, G. Topical Retinoids in Inflammatory Acne: A Retrospective, Investigator-Blinded, Vehicle-Controlled, Photographic Assessment. Clin. Ther. 2005, 27, 216–224. [Google Scholar] [CrossRef]
- Piskin, S.; Uzunali, E. A Review of the Use of Adapalene for the Treatment of Acne Vulgaris. Ther. Clin. Risk Manag. 2007, 3, 621–624. [Google Scholar]
- Irby, C.E.; Yentzer, B.A.; Feldman, S.R. A Review of Adapalene in the Treatment of Acne Vulgaris. J. Adolesc. Health 2008, 43, 421–424. [Google Scholar] [CrossRef]
- Ali, S.; Rawat, N.; Alam, M.; Husain, A. A Review on a Third Generation Retinoidal Agent: Adapalene. Medicine 2016, 2, 11. [Google Scholar]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A Journey from the Molecular Structures and Mechanisms of Action to Clinical Uses in Dermatology and Adverse Effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Uehara, A.; Abe, M.; Shimizu, A.; Motegi, S.; Amano, H.; Ishikawa, O. Successful Treatment of Lichen Spinulosus with Topical Adapalene. Eur. J. Dermatol. 2015, 25, 490–491. [Google Scholar] [CrossRef]
- Treesirichod, A.; Chaithirayanon, S.; Wongjitrat, N.; Wattanapan, P. The Efficacy of Topical 0.1% Adapalene Gel for Use in the Treatment of Childhood Acanthosis Nigricans: A Pilot Study. Indian J. Dermatol. 2015, 60, 103. [Google Scholar] [CrossRef]
- Gupta, R. Plantar warts treated with topical adapalene. Indian J. Dermatol. 2011, 56, 513–514. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, S. Topical Adapalene in the Treatment of Plantar Warts; Randomized Comparative Open Trial in Comparison with Cryo-Therapy. Indian J. Dermatol. 2015, 60, 102. [Google Scholar] [CrossRef]
- Unal, M. Use of Adapalene in Alopecia Areata: Efficacy and Safety of Mometasone Furoate 0.1% Cream versus Combination of Mometasone Furoate 0.1% Cream and Adapalene 0.1% Gel in Alopecia Areata. Dermatol. Ther. 2018, 31, e12574. [Google Scholar] [CrossRef] [PubMed]
- Herane, M.I.; Orlandi, C.; Zegpi, E.; Valdés, P.; Ancić, X. Clinical Efficacy of Adapalene (Differin(®)) 0.3% Gel in Chilean Women with Cutaneous Photoaging. J. Dermatol. Treat. 2012, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ocker, M.; Herold, C.; Ganslmayer, M.; Hahn, E.G.; Schuppan, D. The Synthetic Retinoid Adapalene Inhibits Proliferation and Induces Apoptosis in Colorectal Cancer Cells in Vitro. Int. J. Cancer 2003, 107, 453–459. [Google Scholar] [CrossRef]
- Ocker, M.; Herold, C.; Ganslmayer, M.; Zopf, S.; Hahn, E.G.; Schuppan, D. Potentiated Anticancer Effects on Hepatoma Cells by the Retinoid Adapalene. Cancer Lett. 2004, 208, 51–58. [Google Scholar] [CrossRef]
- Huryn, D.M.; Wipf, P. Chapter 3—Natural Product Chemistry and Cancer Drug Discovery. In Cancer Drug Design and Discovery (Second Edition); Neidle, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 91–120. [Google Scholar]
- Li, H.; Wang, C.; Li, L.; Bu, W.; Zhang, M.; Wei, J.; Tao, L.; Qian, K.; Ma, P. Adapalene Suppressed the Proliferation of Melanoma Cells by S-Phase Arrest and Subsequent Apoptosis via Induction of DNA Damage. Eur. J. Pharmacol. 2019, 851, 174–185. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Ma, P.; Sun, J.; Li, L.; Wei, J.; Tao, L.; Qian, K. The Third-Generation Retinoid Adapalene Triggered DNA Damage to Induce S-Phase Arrest in HaCat Cells. Fundam. Clin. Pharmacol. 2020, 34, 380–388. [Google Scholar] [CrossRef]
- Janani, S.K.; Sureshkumar, R.; Upadhyayula, S.S.N.; Karthika, C.; Vasanthi, C. Will the Polyphenol and Adapalene Combination Be a Good Strategy on Acne Vulgaris? Med. Hypotheses 2019, 133, 109409. [Google Scholar] [CrossRef]
- Bakr, E.; Abdo, H.; Abd-Elaziz, H.; Abd-Elrazek, H.; Amer, M. Adapalene Gel 0.1% vs Ketoconazole Cream 2% and Their Combination in Treatment of Pityriasis Versicolor: A Randomized Clinical Study. Dermatol. Ther. 2020, 33, e13319. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Shalita, A.R.; Yamauchi, P.S.; Dawson, C.; Arsonnaud, S.; Kang, S.; Differin Study Group. Combination Therapy with Adapalene Gel 0.1% and Doxycycline for Severe Acne Vulgaris: A Multicenter, Investigator-Blind, Randomized, Controlled Study. Skinmed 2005, 4, 138–146. [Google Scholar]
- Hayashi, N.; Kawashima, M. Multicenter Randomized Controlled Trial on Combination Therapy with 0.1% Adapalene Gel and Oral Antibiotics for Acne Vulgaris: Comparison of the Efficacy of Adapalene Gel Alone and in Combination with Oral Faropenem. J. Dermatol. 2012, 39, 511–515. [Google Scholar] [CrossRef]
- Wolf, J.E. Potential Anti-Inflammatory Effects of Topical Retinoids and Retinoid Analogues. Adv. Ther. 2002, 19, 109–118. [Google Scholar] [CrossRef]
- Cheng, A.V.; Kim, W.; Escobar, I.E.; Mylonakis, E.; Wuest, W.M. Structure–Activity Relationship and Anticancer Profile of Second-Generation Anti-MRSA Synthetic Retinoids. ACS Med. Chem. Lett. 2020, 11, 393–397. [Google Scholar] [CrossRef]
- Ruamrak, C.; Lourith, N.; Natakankitkul, S. Comparison of Clinical Efficacies of Sodium Ascorbyl Phosphate, Retinol and Their Combination in Acne Treatment. Int. J. Cosmet. Sci. 2009, 31, 41–46. [Google Scholar] [CrossRef] [PubMed]
- DrugBank. Alitretinoin. Available online: https://www.drugbank.ca/drugs/DB00523 (accessed on 2 July 2020).
- PubChem. Alitretinoin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/449171 (accessed on 2 July 2020).
- DrugBank. Acitretin. Available online: https://www.drugbank.ca/drugs/DB00459 (accessed on 2 July 2020).
- PubChem. Acitretin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5284513 (accessed on 10 August 2020).
- Nagpal, S.; Chandraratna, R.A. Recent Developments in Receptor-Selective Retinoids. Curr. Pharm. Des. 2000, 6, 919–931. [Google Scholar] [CrossRef]
- Chandraratna, R.A. Tazarotene: The First Receptor-Selective Topical Retinoid for the Treatment of Psoriasis. J. Am. Acad. Dermatol. 1997, 37, S12–S17. [Google Scholar] [CrossRef]
- Thoreau, E.; Arlabosse, J.-M.; Bouix-Peter, C.; Chambon, S.; Chantalat, L.; Daver, S.; Dumais, L.; Duvert, G.; Feret, A.; Ouvry, G.; et al. Structure-Based Design of Trifarotene (CD5789), a Potent and Selective RARγ Agonist for the Treatment of Acne. Bioorg. Med. Chem. Lett. 2018, 28, 1736–1741. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, S.; Rho, H.S.; Kim, D.H.; Chang, I.S.; Chung, J.H. The Effects of a Novel Synthetic Retinoid, Seletinoid G, on the Expression of Extracellular Matrix Proteins in Aged Human Skin in Vivo. Clin. Chim. Acta 2005, 362, 161–169. [Google Scholar] [CrossRef]
- Lee, E.-S.; Ahn, Y.; Bae, I.-H.; Min, D.; Park, N.H.; Jung, W.; Kim, S.-H.; Hong, Y.D.; Park, W.S.; Lee, C.S. Synthetic Retinoid Seletinoid G Improves Skin Barrier Function through Wound Healing and Collagen Realignment in Human Skin Equivalents. Int. J. Mol. Sci. 2020, 21, 3198. [Google Scholar] [CrossRef]
- Shroot, B.; Michel, S. Pharmacology and Chemistry of Adapalene. J. Am. Acad. Dermatol. 1997, 36, S96–S103. [Google Scholar] [CrossRef]
- WHOCC-ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/?showdescription=yes&code=D10AD03 (accessed on 16 June 2020).
- Latter, G.; Grice, J.E.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Targeted Topical Delivery of Retinoids in the Management of Acne Vulgaris: Current Formulations and Novel Delivery Systems. Pharmaceutics 2019, 11, 490. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopoea, 10th ed.; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2020; p. 1761.
- DrugBank. Adapalene. Available online: https://www.drugbank.ca/drugs/DB00210 (accessed on 10 August 2020).
- Bhatia, G.; Zhou, Y.; Banga, A.K. Adapalene Microemulsion for Transfollicular Drug Delivery. J. Pharm. Sci. 2013, 102, 2622–2631. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Adapalene A7486. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/a7486 (accessed on 10 August 2020).
- Sato, T.; Akimoto, N.; Kitamura, K.; Kurihara, H.; Hayashi, N.; Ito, A. Adapalene Suppresses Sebum Accumulation via the Inhibition of Triacylglycerol Biosynthesis and Perilipin Expression in Differentiated Hamster Sebocytes in Vitro. J. Dermatol. Sci. 2013, 70, 204–210. [Google Scholar] [CrossRef]
- Leid, M. Retinoids. In Burger’s Medicinal Chemistry and Drug Discovery Volume 4: Autocoids, Diagnostics, and Drugs from New Biology, 6th ed.; Abraham, D.J., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003; pp. 317–358. [Google Scholar]
- Chandraratna, R.A.S. Rational Design of Receptor-Selective Retinoids. J. Am. Acad. Dermatol. 1998, 39 (Suppl. 4), S124–S128. [Google Scholar] [CrossRef]
- PubChem. Adapalene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/60164 (accessed on 8 July 2020).
- ChemAxon. Stereoisomer Enumerator. Available online: https://disco.chemaxon.com/calculators/demo/plugins/stereoisomers/ (accessed on 1 July 2020).
- Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics 2020, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A New Class of Synthetic Retinoid Antibiotics Effective against Bacterial Persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; She, P.; Zhou, L.; Liu, Y.; Chen, L.; Luo, Z.; Wu, Y. Bactericidal and Anti-Biofilm Activity of the Retinoid Compound CD437 Against Enterococcus Faecalis. Front. Microbiol. 2019, 10, 2301. [Google Scholar] [CrossRef]
- Garattini, E.; Parrella, E.; Diomede, L.; Gianni’, M.; Kalac, Y.; Merlini, L.; Simoni, D.; Zanier, R.; Ferrara, F.F.; Chiarucci, I.; et al. ST1926, a Novel and Orally Active Retinoid-Related Molecule Inducing Apoptosis in Myeloid Leukemia Cells: Modulation of Intracellular Calcium Homeostasis. Blood 2004, 103, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Valli, C.; Paroni, G.; Francesco, A.M.D.; Riccardi, R.; Tavecchio, M.; Erba, E.; Boldetti, A.; Gianni’, M.; Fratelli, M.; Pisano, C.; et al. Atypical Retinoids ST1926 and CD437 Are S-Phase-Specific Agents Causing DNA Double-Strand Breaks: Significance for the Cytotoxic and Antiproliferative Activity. Mol. Cancer Ther. 2008, 7, 2941–2954. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Samad, R.; Aouad, P.; Gali-Muhtasib, H.; Sweidan, Z.; Hmadi, R.; Kadara, H.; D’Andrea, E.L.; Fucci, A.; Pisano, C.; Darwiche, N. Mechanism of Action of the Atypical Retinoid ST1926 in Colorectal Cancer: DNA Damage and DNA Polymerase α. Am. J. Cancer Res. 2018, 8, 39–55. [Google Scholar]
- Liew, S.K.; Malagobadan, S.; Arshad, N.M.; Nagoor, N.H. A Review of the Structure—Activity Relationship of Natural and Synthetic Antimetastatic Compounds. Biomolecules 2020, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Rosso, J.Q.D. Managing Acne with Adapalene 0.1% and 0.3% Gels—Introduction. J. Drugs Dermatol. 2008, 7, S2. [Google Scholar] [PubMed]
- Thiboutot, D.M.; Gollnick, H.P. Treatment Considerations for Inflammatory Acne: Clinical Evidence for Adapalene 0.1% in Combination Therapies. J. Drugs Dermatol. 2006, 5, 785–794. [Google Scholar] [PubMed]
- Berson, D.; Alexis, A. Adapalene 0.3% for the Treatment of Acne in Women. J. Clin. Aesthet. Dermatol. 2013, 6, 32–35. [Google Scholar] [PubMed]
- Akdeniz, N.; Çalka, Ö.; Özbek, H.; Metin, A. Anti-Inflammatory Effects of Tretinoin (All-Trans-Retinoic Acid) 0.1% and Adapalene 0.1% in Rats. Clin. Exp. Dermat. 2005, 30, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Chayahara, N.; Mukohara, T.; Tachihara, M.; Fujishima, Y.; Fukunaga, A.; Washio, K.; Yamamoto, M.; Nakata, K.; Kobayashi, K.; Takenaka, K.; et al. Adapalene Gel 0.1% Versus Placebo as Prophylaxis for Anti-Epidermal Growth Factor Receptor-Induced Acne-Like Rash: A Randomized Left-Right Comparative Evaluation (APPEARANCE). Oncologist 2019, 24, 885-e413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, D.G.; Sampson, A.P. 49—Skin Disorders. In Medical Pharmacology and Therapeutics, 5th ed.; Waller, D.G., Sampson, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 561–568. [Google Scholar]
- Baran, R.; Maibach, H.I. Textbook of Cosmetic Dermatology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bhalekar, M.; Upadhaya, P.; Madgulkar, A. Formulation and Evaluation of Adapalene-Loaded Nanoparticulates for Epidermal Localization. Drug Deliv. Transl. Res. 2015, 5, 585–595. [Google Scholar] [CrossRef]
- Czernielewski, J.; Michel, S.; Bouclier, M.; Baker, M.; Hensby, J.C. Adapalene Biochemistry and the Evolution of a New Topical Retinoid for Treatment of Acne. J. Eur. Acad. Dermatol. Venereol. 2001, 15 (Suppl. 3), 5–12. [Google Scholar] [CrossRef]
- Jones, D.A. The Potential Immunomodulatory Effects of Topical Retinoids. Dermat. Online J. 2005, 11, 3. [Google Scholar]
- Fakhouri, T.; Yentzer, B.; Kiracofe, E.; Feldman, S.; Salem, W. The Usefulness of Adapalene Outside of Acne Vulgaris. J. Am. Acad. Dermatol. 2011, 64 (Suppl. 1), 19. [Google Scholar]
- Valins, W.; Amini, S.; Berman, B. The Expression of Toll-like Receptors in Dermatological Diseases and the Therapeutic Effect of Current and Newer Topical Toll-like Receptor Modulators. J. Clin. Aesthet. Dermatol. 2010, 3, 20–29. [Google Scholar]
- Kassuga, L.E.d.B.P.; Medrado, M.M.; Chevrand, N.S.; Salles, S.d.A.N.; Vilar, E.G. Fox-Fordyce Disease: Response to Adapalene 0.1%. An. Brasil. Dermatol. 2012, 87, 329–331. [Google Scholar] [CrossRef] [Green Version]
- DiSilvestro, P.A.; DiSilvestro, J.M.; Lernhardt, W.; Pfahl, M.; Mannel, R.S. Treatment of Cervical Intraepithelial Neoplasia Levels 2 and 3 with Adapalene, a Retinoid-Related Molecule. J. Low Genit. Tract Dis. 2001, 5, 33–37. [Google Scholar]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation Disorders: Diagnosis and Management. Am. Fam. Physician 2017, 96, 797–804. [Google Scholar]
- Abe, M.; Inoue, C.; Yokoyama, Y.; Ishikawa, O. Successful Treatment of Darier’s Disease with Adapalene Gel. Pediatr. Dermatol. 2011, 28, 197–198. [Google Scholar] [CrossRef]
- Casals, M.; Campoy, A.; Aspiolea, F.; Carrasco, M.A.; Camps, A. Successful Treatment of Linear Darier’s Disease with Topical Adapalene. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 237–238. [Google Scholar] [CrossRef]
- English, J.C.; Browne, J.; Halbach, D.P. Effective Treatment of Localized Darier’s Disease with Adapalene 0.1% Gel. Cutis 1999, 63, 227–230. [Google Scholar] [PubMed]
- Barzegar, M.; Mozafari, N.A. New Site of Milia En Plaque: Report of a Case and Review of the Literature. Int. J. Dermat. 2015, 54, 1423–1425. [Google Scholar] [CrossRef]
- Altomare, G.; Capella, G.L.; Fracchiolla, C.; Frigerio, E. Effectiveness of Topical Adapalene in Dowling-Degos Disease. Dermatology 1999, 198, 176–177. [Google Scholar] [CrossRef]
- Ogawa, M.; Akiyama, M. Successful Topical Adapalene Treatment for the Facial Lesions of an Adolescent Case of Epidermolytic Ichthyosis. J. Am. Acad. Dermatol. 2014, 71, E103–E105. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.-H.; Cai, Y.-L.; Chuang, H.-C.; Lee, C.-Y.; Lin, Y.-C.; Chiang, C.-P. Application of Ultrasound-Mediated Adapalene-Coated Lysozyme-Shelled Microbubbles in UVA-Induced Skin Photoaging. PLoS ONE 2020, 15, e0232617. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, L.; Zito, P.M. Adapalene. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cantrell, W.C.; Elewksi, B.E. Can Pityriasis Versicolor Be Treated with 2% Ketoconazole Foam? J. Drugs Dermatol. 2014, 13, 855–859. [Google Scholar] [PubMed]
- Shi, T.W.; Ren, X.K.; Yu, H.X.; Tang, Y.B. Roles of Adapalene in the Treatment of Pityriasis Versicolor. Dermatology 2012, 224, 184–188. [Google Scholar] [CrossRef]
- Shah, P.R.; Esaa, F.S.; Gupta, P.; Mercurio, M.G. Trichodysplasia Spinulosa Successfully Treated with Adapalene 0.1% Gel and Oral Valganciclovir in a Renal Transplant Recipient. JAAD Case Rep. 2020, 6, 23–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minucci, S.; Pelicci, P.G. Retinoid Receptors in Health and Disease: Co-Regulators and the Chromatin Connection. Semin. Cell Dev. Biol. 1999, 10, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-N.; Li, H.; Yao, H.; Liu, X.; Li, L.; Leung, K.S.; Kung, H.F.; Lin, M.C.M. Adapalene Inhibits the Activity of Cyclin-Dependent Kinase 2 in Colorectal Carcinoma. Mol. Med. Rep. 2015, 12, 6501–6508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhang, Q.; Luan, S.; Yang, K.; Zheng, M.; Li, K.; Chen, L.; Li, H. Adapalene Inhibits Ovarian Cancer ES-2 Cells Growth by Targeting Glutamic-Oxaloacetic Transaminase 1. Bioorg. Chem. 2019, 93, 103315. [Google Scholar] [CrossRef]
- Medina, D.X.; Chung, E.P.; Bowser, R.; Sirianni, R.W. Lipid and Polymer Blended Polyester Nanoparticles Loaded with Adapalene for Activation of Retinoid Signaling in the CNS Following Intravenous Administration. J. Drug Deliv. Sci. Technol. 2019, 52, 927–933. [Google Scholar] [CrossRef]
- Najafi-Taher, R.; Ghaemi, B.; Amani, A. Delivery of Adapalene Using a Novel Topical Gel Based on Tea Tree Oil Nano-Emulsion: Permeation, Antibacterial and Safety Assessments. Eur. J. Pharm. Sci. 2018, 120, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Sugiyama, M.; Fukunaga, A.; Nishigori, C. Acquired Idiopathic Partial Anhidrosis Successfully Treated with Adapalene Gel. J. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- FDA Center for Drug Evaluation and Research, 22-502 Pharmacology/Toxicology Review and Evaluation. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022502s000PharmR.pdf (accessed on 10 August 2020).
- Weiss, J.S.; Thiboutot, D.M.; Hwa, J.; Liu, Y.; Graeber, M. Long-Term Safety and Efficacy Study of Adapalene 0.3% Gel. J. Drugs Dermatol. 2008, 7 (Suppl. 6), s24–s28. [Google Scholar]
- Otlewska, A.; Baran, W.; Batycka-Baran, A. Adverse Events Related to Topical Drug Treatments for Acne Vulgaris. Expert Opin. Drug Saf. 2020, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Queille-Roussel, C.; Poncet, M.; Mesaros, S.; Clucas, A.; Baker, M.; Soloff, A.M. Comparison of the Cumulative Irritation Potential of Adapalene Gel and Cream with That of Erythromycin/Tretinoin Solution and Gel and Erythromycin/Isotretinoin Gel. Clin. Ther. 2001, 23, 205–212. [Google Scholar] [CrossRef]
- Differin (Adapalene) Gel Label Revised 02/2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021753s004lbl.pdf (accessed on 10 August 2020).
- Herndon, J.H.J.; Stephens, T.J.; Trookman, N.S.; Rizer, R.L.; Preston, N.; Caveney, S.; Gottschalk, R.W. A Comparison of the Tolerability of Adapalene 0.1% Cream and Adapalene 0.1% Lotion in Healthy Individuals. Skinmed 2012, 10, 136–142. [Google Scholar] [PubMed]
- Rao, G.R.R.; Ghosh, S.; Dhurat, R.; Sharma, A.; Dongre, P.; Baliga, V.P. Efficacy, Safety, and Tolerability of Microsphere Adapalene vs. Conventional Adapalene for Acne Vulgaris. Int. J. Dermatol. 2009, 48, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Jo, R.; Kobayashi, Y.; Tsuboi, R.; Okubo, Y. Allergic Contact Dermatitis Caused by Adapalene. Contact Dermat. 2015, 73, 187–188. [Google Scholar] [CrossRef] [PubMed]
- EMA/165360/2018 Updated Measures for Pregnancy Prevention during Retinoid Use 23/03/2018. Available online: https://www.ema.europa.eu/en/documents/referral/retinoid-article-31-referral-updated-measures-pregnancy-prevention-during-retinoid-use_en-0.pdf (accessed on 10 August 2020).
- Williams, A.L.; Campetella, S.; Desesso, J.M. Retinoid Teratogenicity: A Critical Evaluation of Contributing Properties for Adapalene. Birth Defects Res. 2019, 111, 518. [Google Scholar]
- Veraldi, S.; Rossi, L.C.; Barbareschi, M. Are Topical Retinoids Teratogenic? G Ital. Dermatol. Venereol. 2016, 151, 700–705. [Google Scholar]
- Dreno, B.; Bissonnette, R.; Gagne-Henley, A.; Barankin, B.; Lynde, C.; Chavda, R.; Kerrouche, N.; Tan, J. Long-Term Effectiveness and Safety of Up to 48 Weeks’ Treatment with Topical Adapalene 0.3%/Benzoyl Peroxide 2.5% Gel in the Prevention and Reduction of Atrophic Acne Scars in Moderate and Severe Facial Acne. Am. J. Clin. Dermatol. 2019, 20, 725–732. [Google Scholar] [CrossRef]
- DuBois, J.; Ong, G.C.W.; Petkar, G.; Almeida, L.M.C.; Chavda, R.; Kerrouche, N.; Alexis, A.F. Patient-Reported Outcomes in Acne Patients With Skin of Color Using Adapalene 0.3%-Benzoyl Peroxide 2.5%: A Prospective Real-World Study. J. Drugs Dermatol. 2019, 18, 514–520. [Google Scholar]
- Jain, G.K.; Ahmed, F.J. Adapalene Pretreatment Increases Follicular Penetration of Clindamycin: In Vitro and in Vivo Studies. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 326–329. [Google Scholar] [CrossRef]
- Vasanth, S.; Dubey, A.; Ravi, G.S.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and Investigation of Vitamin C-Enriched Adapalene-Loaded Transfersome Gel: A Collegial Approach for the Treatment of Acne Vulgaris. AAPS PharmSciTech 2020, 21, 61. [Google Scholar] [CrossRef]
- Neumeister, C.; Boedeker, R.H.; Borelli, C.; Schwantes, U. Acne Vulgaris in Adolescents: Parallel Topical Treatment with Nadifloxacin and Adapalene. N S Arch. Pharmacol. 2020, 393, 40. [Google Scholar]
- Wilhelm, K.P.; Wilhelm, D.; Neumeister, C.; Zsolt, I.; Schwantes, U. Lack of Irritative Potential of Nadifloxacin 1% When Combined with Other Topical Anti-Acne Agents. Clin. Exp. Dermatol. 2012, 37, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nakagawa, T.; Fukamachi, K.; Nakamura, M.; Tokura, Y. Efficacy of Combined Topical Treatment of Acne Vulgaris with Adapalene and Nadifloxacin: A Randomized Study. J. Dermatol. 2011, 38, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.M.; Weiss, J.; Bucko, A.; Eichenfield, L.; Jones, T.; Clark, S.; Liu, Y.; Graeber, M.; Kang, S. Adapalene-Benzoyl Peroxide, a Fixed-Dose Combination for the Treatment of Acne Vulgaris: Results of a Multicenter, Randomized Double-Blind, Controlled Study. J. Am. Acad. Dermatol. 2007, 57, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Jiang, X. Effects of Adapalene-Benzoyl Peroxide Combination Gel in Treatment or Maintenance Therapy of Moderate or Severe Acne Vulgaris: A Meta-Analysis. Ann. Dermatol. 2014, 26, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kardeh, S.; Saki, N.; Jowkar, F.; Kardeh, B.; Moein, S.A.; Khorraminejad-Shirazi, M.H. Efficacy of Azithromycin in Treatment of Acne Vulgaris: A Mini Review. World J. Plast. Surg. 2019, 8, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouloc, A.; Roo, E.; Imko-Walczuk, B.; Moga, A.; Chadoutaud, B.; Dréno, B. A Skincare Combined with Combination of Adapalene and Benzoyl Peroxide Provides a Significant Adjunctive Efficacy and Local Tolerance Benefit in Adult Women with Mild Acne. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1727–1731. [Google Scholar] [CrossRef]
- Kamoji, S.G.; Huggi, G.; Pise, G.A.; Nayak, J.J.; Dastikop, S.V.; Patil, M.N. A Double Blind Randomized Study to Compare Efficacy of 5% Dapsone Gel vs Combination of Adapalene-Clindamycin Gel in the Treatment of Mild to Moderate Acne Vulgaris. J. Dermatol. Cosmet. 2018, 2, 202–205. [Google Scholar]
- Chlebus, E.; Serafin, M.; Chlebus, M. Is Maintenance Treatment in Adult Acne Important? Benefits from Maintenance Therapy with Adapalene, and Low Doses of Alpha and Beta Hydroxy Acids. J. Dermatol. Treat. 2019, 30, 568–571. [Google Scholar] [CrossRef]
- Kayhan, S.; Sabuncu, İ.; Saraçoğlu, Z.N.; Aksu, A.E.K.; Tozun, M. Comparison of Safety and Efficacy of Oral Azithromycin-Topical Adapalene Versus Oral Doxycycline-Topical Adapalene in the Treatment of Acne Vulgaris and Determination of the Effects of These Treatments on Patients’ Quality of Life. Turkderm 2012, 46, 151–155. [Google Scholar]
- Wahab, M.A.; Rahman, M.H.; Monamie, N.S.; Jamaluddin, M.; Khondker, L.; Afroz, W. Isotretinoin versus Weekly Pulse Dose Azithromycin in the Treatment of Acne-a Comparative Study. J. Pak. Assoc. Dermatol. 2016, 18, 9–14. [Google Scholar]
- Mukherjee, R.; Davies, P.J.A.; Crombie, D.L.; Bischoff, E.D.; Cesario, R.M.; Jow, L.; Hamann, L.G.; Boehm, M.F.; Mondon, C.E.; Nadzan, A.M.; et al. Sensitization of Diabetic and Obese Mice to Insulin by Retinoid X Receptor Agonists. Nature 1997, 386, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.; Bourguet, W.; Gronemeyer, H.; Lera, A.R. Modulation of RXR Function through Ligand Design. Biochim. Biophys. Acta 2012, 1821, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Souto, J.A.; Lera, A.R. Ligand Design for Modulation of RXR Functions. Methods Mol. Biol. 2019, 2019, 51–72. [Google Scholar] [PubMed]
- Yentzer, B.A.; Loyd, A.; Jorizzo, J.L. Adapalene Gel 0.3%: A Novel Topical Retinoid Formulation for Acne Vulgaris. Exp. Rev. Dermatol. 2008, 3, 161–165. [Google Scholar] [CrossRef]
- Mokhtari, F.; Shajari, A.; Iraji, F.; Faghihi, G.; Siadat, A.H.; Sadeghian, G.; Adibi, N. The Effectiveness of Adapalene 0.1% with Intense Pulsed Light versus Benzoyl Peroxide 5% with Intense Pulsed Light in the Treatment of Acne Vulgaris: A Comparative Study. J. Res. Med. Sci. 2019, 24, 101. [Google Scholar]
- Sift, B.; Naeem, M.A.; Shahiq-uz-Zaman; Masood-ur-Rehman; Liaqat, A.; Khaleeq, A.; Hina, H. Formulation of Adapalene Emulgel and Its Optimization for Its Potential Topical Application. Lat. Am. J. Pharm. 2020, 39, 694–700. [Google Scholar]
- Pajic, N.B.; Ilic, T.; Nikolic, I.; Dobricic, V.; Pantelic, I.; Savic, S. Alkyl Polyglucoside-Based Adapalene-Loaded Microemulsions for Targeted Dermal Delivery: Structure, Stability and Comparative Biopharmaceutical Characterization with a Conventional Dosage Form. J. Drug Deliv. Sci. Technol. 2019, 54, 1245. [Google Scholar]
- Nadal, J.M.; Camargo, G.d.A.; Novatski, A.; Macenhan, W.R.; Dias, D.T.; Barboza, F.M.; Lyra, A.; Roik, J.R.; de Paula, J.P.; Somer, A.; et al. Adapalene-Loaded Poly(ε-Caprolactone) Microparticles: Physicochemical Characterization and in Vitro Penetration by Photoacoustic Spectroscopy. PLoS ONE 2019, 14, e0213625. [Google Scholar] [CrossRef] [Green Version]
- Sallam, M.A.; Boscá, M.T.M. Mechanistic Analysis of Human Skin Distribution and Follicular Targeting of Adapalene-Loaded Biodegradable Nanospheres With an Insight Into Hydrogel Matrix Influence, In Vitro Skin Irritation, and In Vivo Tolerability. J. Pharm. Sci. 2017, 106, 3140–3149. [Google Scholar] [CrossRef]
- Najafi-Taher, R.; Amani, A. Nanoemulsions: Colloidal Topical Delivery Systems for Antiacne Agents- A Mini-Review. Nanomed. Res. J. 2017, 2, 49–56. [Google Scholar]
- Jain, A.K.; Jain, A.; Garg, N.K.; Agarwal, A.; Jain, A.; Jain, S.A.; Tyagi, R.K.; Jain, R.K.; Agrawal, H.; Agrawal, G.P. Adapalene Loaded Solid Lipid Nanoparticles Gel: An Effective Approach for Acne Treatment. Colloids Surf. B Biointerfaces 2014, 121, 222–229. [Google Scholar] [CrossRef]
- Guo, C.; Khengar, R.H.; Sun, M.; Wang, Z.; Fan, A.; Zhao, Y. Acid-Responsive Polymeric Nanocarriers for Topical Adapalene Delivery. Pharm. Res. 2014, 31, 3051–3059. [Google Scholar] [CrossRef]
- Ramezanli, T.; Zhang, Z.; Michniak-Kohn, B.B. Development and Characterization of Polymeric Nanoparticle-Based Formulation of Adapalene for Topical Acne Therapy. Nanomed. Nanotechnol. 2017, 13, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kandekar, S.G.; Río-Sancho, S.D.; Lapteva, M.; Kalia, Y.N. Selective Delivery of Adapalene to the Human Hair Follicle under Finite Dose Conditions Using Polymeric Micelle Nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kale, D.P.; Swami, R.; Katiyar, S.S. Codelivery of Benzoyl Peroxide & Adapalene Using Modified Liposomal Gel for Improved Acne Therapy. Nanomedicine (Lond.) 2018, 13, 1481–1493. [Google Scholar] [PubMed]
- Brammann, C.; Mueller-Goymann, C.C. Incorporation of Benzoyl Peroxide Nanocrystals into Adapalene-Loaded Solid Lipid Microparticles: Part I—Nanocrystalline Benzoyl Peroxide. Int. J. Pharm. 2019, 564, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Brammann, C.; Müller-Goymann, C.C. Incorporation of Benzoyl Peroxide Nanocrystals into Adapalene-Loaded Solid Lipid Microparticles: Part II—Solid-in-Oil Dispersion of Nanoparticulate Benzoyl Peroxide. Int. J. Pharm. 2019, 572, 118792. [Google Scholar] [CrossRef]
- Dubey, A. Niosomal Gel of Adapalene: Its Formulation, Physicochemical Properties and Evaluation for Mild-Acne. Adv. Biomed. Pharm. 2015, 2, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Banga, A.K. Intradermal and Follicular Delivery of Adapalene Liposomes. Drug Dev. Ind. Pharm. 2016, 42, 871–879. [Google Scholar] [CrossRef]
- Jain, A.; Garg, N.K.; Jain, A.; Kesharwani, P.; Jain, A.K.; Nirbhavane, P.; Tyagi, R.K. A Synergistic Approach of Adapalene-Loaded Nanostructured Lipid Carriers, and Vitamin C Co-Administration for Treating Acne. Drug Dev. Ind. Pharm. 2016, 42, 897–905. [Google Scholar] [CrossRef]

| Retinoid Generation | Administration/Indications | Representatives | Chemical Structure | Receptor(s) | References |
|---|---|---|---|---|---|
| I | Systemic and topical/acne vulgaris, photoaging, cosmetic ingredient | Retinol (vitamin A) |  | - | [33] |
| Topical/acne vulgaris, photoaging | Tretinoin (all-trans-retinoic acid; vitamin A acid) |  | RAR-α, RAR-β, RAR-γ; RXR | [4,8] | |
| Systemic and topic/acne vulgaris | Isotretinoin (13-cis-retinoic acid) |  | No clear receptor affinity | [4] | |
| Systemic and topical/Karposi sarcoma | Alitretinoin (9-cis-retinoic acid) |  | RAR-α, RAR-β, RAR-γ | [14,34,35] | |
| II | Systemic/psoriasis | Acitretin |  | RAR-α, RAR-β, RAR-γ | [36,37] |
| Topical/acne vulgaris | Motretinide |  | - | [4] | |
| III | Topical/acne vulgaris, psoriasis | Tazarotene |  | RAR-β, RAR-γ | [4,8,38,39] |
| Systemic and topical/cutaneous T cell lymphomas | Bexarotene |  | RXR | [24] | |
| Topical/acne vulgaris | Adapalene |  | RAR-β, RAR-γ | [4,8,38] | |
| IV | Topical/acne vulgaris (facial and truncal) | Trifarotene |  | RAR-γ | [8,9,40] |
| -/Photo-aging wound healing | Seletinoid G |  | RAR-γ | [41,42] |
| Property | Description | References |
|---|---|---|
| IUPAC name | 6-[3-(1-adamantyl)-4-methoxyphenyl]naphthalene-2-carboxylic acid | [43] |
| CAS number | 106685-40-9 | |
| ATC code | D10AD03 (retinoids for topical use group) | [44] |
| Molecular formula | C28H28O3 | [43] |
| Molecular weight (MW) | 412.52 g/mol | [45,46] |
| Appearance | White or almost white powder | |
| Solubility | Soluble in dimethyl sulfoxide (DMSO) (>10 mg/mL at 25 °C), dimethylformamide (DMF) (5 mg/mL at 25 °C) and tetrahydrofuran; sparingly soluble in ethanol (<1 mg/mL at 25 °C), and practically insoluble in water. | [14,46] |
| Melting point | 319–322 °C | [14,47] |
| Boiling point | 606.3 °C at 760 mmHg | |
| Density | 1.2 g/cm3 | [14] |
| Refractive index | 1.66 | |
| pKa | 4.23; 3.99 (strongest acidic), −4.8 (strongest basic) | [14,45,47,48] |
| Lipophilic parameters | log P: 8.04, 8.6; 6.06, 6.47 AlogP: 6.68 XlogP: 7.7 | [45,47,48] |
| Storage temperature | 2–8 °C | [49] |
| Biologic Effect | Condition | References |
|---|---|---|
| Anti-inflammatory | Rosacea (reduction in inflammatory papules) Inflammatory dermatoses Fox–Fordyce disease Alopecia areata | [15,72] [20,74] |
| Immunomodulatory properties | Cervical intraepithelial neoplasia Actinic keratoses Actinic keratoses in solid organ transplant Pigmentary disorders Alopecia areata Plantar warts | [75] [15] [76] [20] [18,19] |
| Keratolytic | Acral Darier disease Milia en plaque Dowling-Degos disease Epidermolytic ichthyosis | [77,78] [79,80] [81,82] [83] |
| Comedolytic | Hyperkeratosis conditions | [82] |
| Depigmenting effect | Acanthosis nigricans | [17] |
| Differentiation and maintenance of hair follicles | Alopecia areata | [20] |
| Removal of melanin Inhibitory action on melanogenesis Potential to promote collagen synthesis | Photoaging | [21] [83] |
| Adverse Reactions | Advice for Patients |
|---|---|
| Phototoxicity | Use sunscreen products. Wear clothes that cover the treated area. Avoid exposure to sunlight or sunlamps (UV light) or minimize it. |
| Environmental exposure | Avoid windy or rainy weather because it may produce local irritation or skin discomfort. |
| Local cutaneous reactions Contact dermatitis | Avoid the use of retinoids if any lesions on the skin are present. Introduce the ADP slowly in the therapeutic routine. |
| Allergic/hypersensitivity reactions (face and eyelid edema, pruritus, and lip swelling) | Stop the treatment if it is necessary. |
| Topical Combination | Therapeutic Use | Pharmaceutical Form | Duration of Treatment | Observations | References | |
|---|---|---|---|---|---|---|
| Content in ADP | Content in Other Active Pharmaceutical Substances (APIs) | |||||
| 0.1% | 2.5% Benzoyl peroxide (Normaderm®, Laboratoires Vichy, France - adjunctive skincare) | Mild acne | Gel for both APIs | 90 days | Human patients ADP and benzoyl peroxide—in the evening Normaderm—in the morning administration | [115] |
| 0.3% | 2.5% Benzoyl peroxide | Atrophic scars in moderate or severe acne vulgaris | Gel | 48 weeks | Human patients | [105] |
| 0.3% | 2.5% Benzoyl peroxide | Skin of color and mild to severe acne vulgaris | Gel | 16 weeks | Human patients | [106] |
| 1% | 1% Clindamycin (phosphate) | Acne | Gel for both APIs | Pretreatment of the skin with ADP gel for 5 min | Excised rat skin Hands of human volunteers | [107] |
| 0.1% | 1% Clindamycin | Mild to moderate acne | Gel for both pharmaceuticals | 4 weeks (applied gel 30 min at night) | Human patients | [116] |
| 0.1% | 2% Ketoconazole | Pityriasis versicolor | Gel (in the morning) Cream (at night) | 4 weeks | Human patients | [28] |
| 0.1% | 0.1% Mometasone (furoate) | Alopecia areata | Cream (mometasone) Gel (ADP) | 12 weeks | Human patients | [20] |
| 0.1% | 1% Nadifloxacin | Moderate to severe acne | Cream (nadifloxacin) Gel (0.1%) | 8 weeks | Human patients ADP—in the evening Nadifloxacin—in the morning, and after ADP in the evening | [111] |
| 0.1% | 0.2% lactic acid, 0.2% glycolic acid, 0.04% citric acid, 0.01% malic acid and 0.001% salicylic acid (active day cream); 0.3% lactic acid, 0.3% glycolic acid, 0.06% citric acid, 0.015% malic acid and 0.0015% salicylic acid (active night cream) | Mild and moderate acne | Gel (ADP) Cream: active day and active night | 12 weeks | Human patients ADP—three times a day in the evening | [117] |
| Oral APIs and Doses | Topical Formulations (ADP and Other APIs Content) | Therapeutic Use | Topical Pharmaceutical Forms | Duration of Treatment | Administration | References |
|---|---|---|---|---|---|---|
| Azithromycin 500 mg/day | 0.1% ADP and 5% benzoyl peroxide | Acne vulgaris | Gel or cream (ADP) Gel (benzoyl peroxide) | 12 weeks | Azithromycin—3 days a week ADP—once daily in the morning Benzoyl peroxide—once daily in the evening | [118] |
| Azithromycin 500 mg/day | 0.1% | Acne vulgaris | Gel | 12 weeks | Azithromycin—3 consecutive days followed by 7 days rest (a 10-day cycle) | [118] |
| Azithromycin 500 mg/day | Erythromycin lotion (not specified%) and then ADP (not specified%) | Moderate and severe acne | Lotion (erythromycin) Not specified (ADP) | 12 weeks 20 weeks | Azithromycin—3 days a week for 3 months | [119] |
| Doxycycline 100 mg/day | Non-specified | Acne vulgaris | Gel | 12 weeks | - | [118] |
| Doxycycline 40 mg mg/day | 0.3% ADP and 2.5% benzoyl peroxide | Severe acne | Gel | 12 weeks | Doxycycline: 30 mg immediate release and 10 mg delayed release beads 25 human patients | [30] |
| Faropenem 600 mg/day | 0.1% | Moderate and severe acne | Gel | 4 weeks | - | [29] |
| Isotretinoin 0.5–1 mg/kg | Erythromycin lotion (not specified%) and then ADP (not specified%) | Moderate and severe acne | Lotion (erythromycin) Not specified (ADP) | 12 weeks 20 weeks | Isotretinoin—5 months | [119] |
| Valganciclovir 450 mg (3 days per week) | 0.1% ADP | Trichodysplasia spinulosa | ADP-gel | 7 weeks | A 25-year-old woman (before kidney transplant) | [87] |
| Pharmaceutical Formulation | Content in ADP | Content in Other API/APIs | Treatment of | Duration of Treatment | Observations | References |
|---|---|---|---|---|---|---|
| Ultrasound-mediated ADP-coated lysozyme-shelled microbubbles | 13.99% ± 0.59% (in coated lysozyme-shelled microbubbles) | - | Photoaging | 5 weeks | Animal model experiment (mice) | [83] |
| Transfersome prepared by reverse-phase evaporation | - | Ascorbic acid 15% w/w | Acne vulgaris | 0, 24 h; 72 h | Animal model experiment (rats) | [108] |
| Solid lipid microparticle (SLM)-dispersion | 0.1% | Benzoyl peroxide 2.5% | Acne vulgaris | - | Porcine ear skin experiment | [135] |
| Niosomal gel | 95.04% ± 0.57% to 90.68% ± 0.39% (in niosomes) | - | Mild acne vulgaris | 7 days | Animal model experiment (albino rats) | [137] |
| Liposomal formulation | 97.01% ± 1.84% w/w encapsulation efficiency | - | Testing skin permeation properties | 15 h | In vitro permeation studies on full-thickness pig ear skin (Franz diffusion cells) | [138] |
| Nanostructured lipid carriers | 87.29% ± 1.6% entrapped efficiency | Ascorbyl-6-palmitate 15% w/w | Testosterone induced acne | 4 weeks | Testosterone induced acne animal model experiment (Wistar rats) | [139] |
| Microemulsion | 0.1% w/v | - | Testing penetration pathways into the skin | 24 h | In vitro transfollicular delivery studies on porcine ear skin (Franz diffusion cells) | [48] |
| Lotion | 0.1% | - | Healthy skin | 3 weeks | Healthy volunteers | [99] |
| Microsphere gel formulation | 0.1% | - | Mild to moderate acne vulgaris | 12 weeks | Human patients | [100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, A.; Tanase, C.; Pascu, G.-A.; Todoran, N. Recent Advances Regarding the Therapeutic Potential of Adapalene. Pharmaceuticals 2020, 13, 217. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13090217
Rusu A, Tanase C, Pascu G-A, Todoran N. Recent Advances Regarding the Therapeutic Potential of Adapalene. Pharmaceuticals. 2020; 13(9):217. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13090217
Chicago/Turabian StyleRusu, Aura, Corneliu Tanase, Georgiana-Andreea Pascu, and Nicoleta Todoran. 2020. "Recent Advances Regarding the Therapeutic Potential of Adapalene" Pharmaceuticals 13, no. 9: 217. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13090217