The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections
Abstract
:1. Introduction
- Andrographis paniculata (Burm. F.) Wall. ex. Nees, Acanthaceae (AP),
- Eleutherococcus senticosus (Rupr. & Maxim.) Maxim, Araliaceae (ES),
- Glycyrrhiza spp., Fabaceae (GS),
- Panax spp., Araliaceae (PS),
- Rhodiola rosea L., Crassulaceae (RR),
- Schisandra chinensis (Turcz.) Bail., Schisandraceae (SC), and
- Withania somnifera (L.) Dunal, Solanaceae (WS)
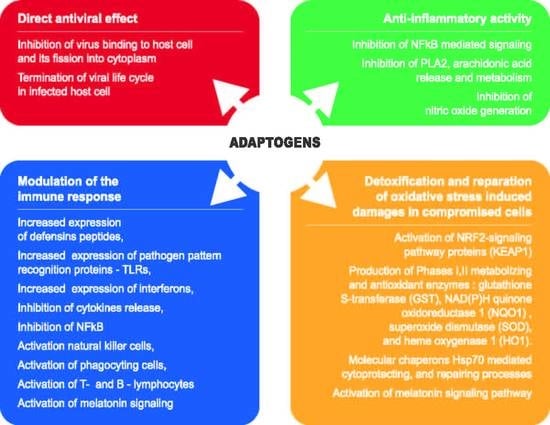
- Specific antiviral action-preventing viruses binding to host cells, and on non-structural (Nsps) and structural proteins involved in viral life cycle in infected host cells and replication of the virus;
- Non-specific antiviral action by the effects on:
- ○
- Innate immunity including activation of defensins, the complement system, upregulation of expression of pathogen’s pattern recognition receptors, specifically TLR, and interferons;
- ○
- Downregulation of expression of pro-inflammatory cytokines IL-1, IL2, IL-6, IL-8, and TNF, activation of natural killer cells, mucous sentinel and phagocyting cells (mast cells, dendritic cells, macrophages, neutrophils, eosinophils, and basophils) and the melatonin signaling pathways;
- ○
- Adaptive immunity including T cells and MHC proteins, B cells and antibodies.
- Anti-inflammatory activity by inhibition of:
- ○
- Release of arachidonic acid from membrane phospholipids following conversion into COX-2 and lipoxygenase-mediated pro-inflammatory metabolites such as prostaglandins, thromboxane B2, leukotrienes, as well as platelet-activating factor;
- ○
- Inducible NO synthase;
- ○
- NF-κB-mediated pro-inflammatory signaling pathways.
- Detoxifying and cytoprotectant activity in oxidative stress-induced injuries of compromised cells and tissues:
- ○
- Activation of the NRF2-mediated oxidative stress response signaling pathway regulated production of chaperons and stress response proteins, activity of phase I and II metabolizing enzymes, phase III detoxifying proteins, proteasomal degradation proteins, antioxidant proteins (superoxide dismutase (SOD), glutathione S-transferase (GST), NAD(P)H quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 (HO1);
- ○
- Activation of expression and release of molecular chaperons Hsp70, which mediate cytoprotectant and repair processes;
- Activation of the melatonin signaling pathways.
- N-terminal gene 1 protein (Nsp1) suppresses host innate immune response, inhibiting type-I interferon production and induces host mRNA degradation [24];
- Nsp3 (papain-like protease, PLpro) is essential for virus replication and to antagonize the host’s innate immunity;
- Nsp5 (3-chimotrypsin-like protease, 3CLpro) mediates viral replication, transcription and the maturation of Nsps, which is essential in the life cycle of the virus;
- Nsp12 (PNA-dependent PNA polymerase enzyme, RdRp) is a conserved vital enzyme of the coronavirus replication/transcription complex;
- Nsp13 (helicase enzyme) is a multifunctional protein necessary for the replication of coronavirus.
2. Results
2.1. Pre-Clinical Investigations
2.2. Clinical Investigations
2.2.1. Andrographis paniculata
2.2.2. Eleutherococcus senticosus
2.2.3. Glycyrrhiza spp.
2.2.4. Panax spp.
2.2.5. Rhodiola rosea
2.2.6. Schisandra chinensis
2.2.7. Withania somnifera
2.2.8. Combination Products
2.2.9. Activation of the Melatonin Signaling Pathway
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.A.; Wolchok, J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Schijns, V.; Lavelle, E.C. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur. J. Immunol. 2020, 50, 932–938. [Google Scholar] [CrossRef]
- Lega, S.; Naviglio, S.; Volpi, S.; Tommasini, A. Recent Insight into SARS-CoV2 Immunopathology and Rationale for Potential Treatment and Preventive Strategies in COVID-19. Vaccines 2020, 8, 224. [Google Scholar] [CrossRef]
- Yang, R.; Liu, H.; Bai, C.; Wang, Y.; Zhang, X.; Guo, R.; Wu, S.; Wang, J.; Leung, E.; Chang, H.; et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol. Res. 2020, 157, 104820. [Google Scholar] [CrossRef]
- Sakurai, A.; Sasaki, T.; Kato, S.; Hayashi, M.; Tsuzuki, S.-I.; Ishihara, T.; Iwata, M.; Morise, Z.; Doi, Y. Natural History of Asymptomatic SARS-CoV-2 Infection. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Innate immunity. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.-J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef]
- Lazarev, N.V.; Ljublina, E.I.; Rozin, M.A. State of nonspecific resistance. Patol. Fiziol. Experim. Ter. 1959, 3, 16–21. [Google Scholar]
- Brekhman, I.; Dardymov, I. New substances of plant origin which increase nonspecific resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Nörr, H.; Winterhoff, H. Plant adaptogens. Phytomedicine 1994, 1, 63–76. [Google Scholar] [CrossRef]
- Yang, Y.M.; Noh, K.; Han, C.Y.; Kim, S.G. Transactivation of Genes Encoding for Phase II Enzymes and Phase III Transporters by Phytochemical Antioxidants. Molecules 2010, 15, 6332–6348. [Google Scholar] [CrossRef] [Green Version]
- Pooladanda, V.; Thatikonda, S.; Godugu, C. The current understanding and potential therapeutic options to combat COVID-19. Life Sci. 2020, 254, 117765. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, E.; Maroufi, P.; Khodadadi, E.; Esposito, I.; Ganbarov, K.; Espsoito, S.; Yousefi, M.; Zeinalzadeh, E.; Kafil, H.S. Study of combining virtual screening and antiviral treatments of the Sars-CoV-2 (Covid-19). Microb. Pathog. 2020, 146, 104241. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef]
- Mirza, M.U.; Froeyen, M. Structural elucidation of SARS-CoV-2 vital proteins: Computational methods reveal potential drug candidates against main protease, Nsp12 polymerase and Nsp13 helicase. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Saber-Ayad, M.; Saleh, M.A.; Abu-Gharbieh, E. The Rationale for Potential Pharmacotherapy of COVID-19. Pharmaceuticals 2020, 13, 96. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fang, Y.; Xu, T.; Ni, W.-J.; Shen, A.-Z.; Meng, X.-M. Potential therapeutic targets and promising drugs for combating SARS-CoV-2. Br. J. Pharmacol. 2020, 177, 3147–3161. [Google Scholar] [CrossRef]
- Kamitani, W.; Narayanan, K.; Huang, C.; Lokugamage, K.; Ikegami, T.; Ito, N.; Kubo, H.; Makino, S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 12885–12890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis Poverty 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Hamid, S.; Mir, M.Y.; Rohela, G.K. Novel coronavirus disease (COVID-19): A pandemic (epidemiology, pathogenesis and potential therapeutics). N. Microbes N. Infect. 2020, 35, 100679. [Google Scholar] [CrossRef]
- Yi, Y.; Lagniton, P.N.P.; Ye, S.; Li, E.; Xu, R.-H. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020, 16, 1753–1766. [Google Scholar] [CrossRef]
- Peron, J.P.S.; Nakaya, H. Susceptibility of the Elderly to SARS-CoV-2 Infection: ACE-2 Overexpression, Shedding, and Antibody-dependent Enhancement (ADE). Clinics 2020, 75. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [Green Version]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J. Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS−Coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008, 22, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Glatthaar-Saalmüller, B.; Sacher, F.; Esperester, A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antivir. Res. 2001, 50, 223–228. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Li, H.-Y.; Liu, X.-Y.; Zhang, F.-M.; Li, X.; Piao, Y.-A.; Xie, Z.-P.; Chen, Z.-H.; Li, X. The anti-respiratory syncytial virus effect of active compound of Glycyrrhiza GD4 in vitro. Zhong Yao Cai 2006, 29, 692–694. [Google Scholar] [PubMed]
- Lee, J.S.; Ko, E.-J.; Hwang, H.S.; Lee, Y.-N.; Kwon, Y.-M.; Kim, M.-C.; Kang, S.-M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int. J. Mol. Med. 2014, 34, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, L.; Wu, W.; Yang, J.; Yang, Z.; Liu, S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes Infect. 2017, 19, 605–615. [Google Scholar] [CrossRef]
- Yu, B.; Dai, C.Q.; Jiang, Z.Y.; Li, E.Q.; Chen, C.; Wu, X.L.; Chen, J.; Liu, Q.; Zhao, C.L.; He, J.X.; et al. Andrographolide as an anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin. J. Integr. Med. 2014, 20, 540–545. [Google Scholar] [CrossRef]
- Ko, H.-C.; Wei, B.-L.; Chiou, W.-F. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J. Ethnopharmacol. 2006, 107, 205–210. [Google Scholar] [CrossRef]
- Fedorov, Y.V.; Vasilyeva, O.A.; Vasilyev, N.V. Effect of some stimulants of plant origin on the development of antibodies and immunomorphological reactions during acarid-borne encephalitis. Cent. Nerv. Syst. Stimul. 1966, 1, 99–105. [Google Scholar]
- Protasova, S.F.; Zykov, M.P. Antiviral effect of Eleutherococcus in experimental influenza infection. In New Data on Eleutherococcus, Proceedings of the II International Symposium on Eleutherococcus, Moscow, USSR, 1984; Far East Academy of Sciences of the USSR: Vladivostok, USSR, 1986; pp. 123–126. [Google Scholar]
- Yan, W.; Chen, J.; Wei, Z.; Wang, X.; Zeng, Z.; Tembo, D.; Wang, Y.; Wang, X. Effect of eleutheroside B1 on non-coding RNAs and protein profiles of influenza A virus-infected A549 cells. Int. J. Mol. Med. 2020, 45, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zheng, C.; He, J.; Zhang, W.; Huang, X.A.; Li, X.; Wang, Y.; Wang, X. Eleutheroside B1 mediates its anti-influenza activity through POLR2A and N-glycosylation. Int. J. Mol. Med. 2018, 42, 2776–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H.J. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antivir. Res. 2009, 83, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-G.; Jin, Y.-H.; Lee, H.; Oh, T.W.; Yim, N.-H.; Cho, W.-K.; Ma, J.Y. Protective Effect of Panax notoginseng Root Water Extract against Influenza A Virus Infection by Enhancing Antiviral Interferon-Mediated Immune Responses and Natural Killer Cell Activity. Front. Immunol. 2017, 8, 1542. [Google Scholar] [CrossRef]
- Dong, W.; Farooqui, A.; Leon, A.J.; Kelvin, D.J. Inhibition of influenza A virus infection by ginsenosides. PLoS ONE 2017, 12, e0171936. [Google Scholar] [CrossRef]
- Kim, E.-H.; Kim, S.-W.; Park, S.-J.; Kim, S.; Yu, K.-M.; Kim, S.G.; Lee, S.H.; Seo, Y.-K.; Cho, N.-H.; Kang, K.; et al. Greater Efficacy of Black Ginseng (CJ EnerG) over Red Ginseng against Lethal Influenza A Virus Infection. Nutrients 2019, 11, 1879. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Hwang, H.S.; Ko, E.-J.; Lee, Y.-N.; Kwon, Y.-M.; Kim, M.-C.; Kang, S.-M. Immunomodulatory Activity of Red Ginseng against Influenza A Virus Infection. Nutrients 2014, 6, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jung, Y.-J.; Kim, K.-H.; Kwon, Y.; Kim, Y.-J.; Zhang, Z.; Kang, H.-S.; Wang, B.-Z.; Quan, F.-S.; Kang, S.-M. Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses. Viruses 2018, 10, 471. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.L.; Kim, H.J.; Choi, Y.R.; Kim, H.-J. Intake of Korean red ginseng extract and saponin enhances the protection conferred by vaccination with inactivated influenza a virus. J. Ginseng Res. 2012, 36, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.Y.; Kim, H.J.; Kim, H.-J. A Comparative Study of the Effects of Whole Red Ginseng Extract and Polysaccharide and Saponin Fractions on Influenza A (H1N1) Virus Infection. Biol. Pharm. Bull. 2013, 36, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Song, J.M.; Quan, F.S.; Park, K.M.; Cho, Y.K.; Kang, S.M. Protective Effect of Korean Red Ginseng Extract on the Infections by H1N1 and H3N2 Influenza Viruses in Mice. J. Med. Food 2012, 15, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorganic Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, T.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob. Agents Chemother. 1997, 41, 551. [Google Scholar] [CrossRef] [Green Version]
- Park, E.H.; Yum, J.; Ku, K.B.; Kim, H.M.; Kang, Y.M.; Kim, J.C.; Kim, J.A.; Kang, Y.K.; Seo, S.H. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J. Ginseng Res. 2014, 38, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sornpet, B.; Potha, T.; Tragoolpua, Y.; Pringproa, K. Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac. J. Trop. Med. 2017, 10, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Ogbomo, H.; Altenbrandt, B.; Leutz, A.; Doerr, H.W.; Cinatl, J. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med. Microbiol. Immunol. 2010, 199, 291–297. [Google Scholar] [CrossRef]
- Panraksa, P.; Ramphan, S.; Khongwichit, S.; Smith, D.R. Activity of andrographolide against dengue virus. Antivir. Res. 2017, 139, 69–78. [Google Scholar] [CrossRef]
- Ramalingam, S.; Karupannan, S.; Padmanaban, P.; Vijayan, S.; Sheriff, K.; Palani, G.; Krishnasamy, K.K. Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. AYU 2018, 39, 87–91. [Google Scholar] [CrossRef]
- Wintachai, P.; Kaur, P.; Lee, R.C.H.; Ramphan, S.; Kuadkitkan, A.; Wikan, N.; Ubol, S.; Roytrakul, S.; Chu, J.J.H.; Smith, D.R. Activity of andrographolide against chikungunya virus infection. Sci. Rep. 2015, 5, 14179. [Google Scholar] [CrossRef] [Green Version]
- Diwaker, D.; Mishra, K.P.; Ganju, L.; Singh, S.B. Rhodiola inhibits dengue virus multiplication by inducing innate immune response genes RIG-I, MDA5 and ISG in human monocytes. Arch. Virol. 2014, 159, 1975–1986. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhou, J.; Sun, X.; Wang, S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 2009, 16, 146–155. [Google Scholar] [CrossRef]
- Jain, J.; Narayanan, V.; Chaturvedi, S.; Pai, S.; Sunil, S. In Vivo Evaluation of Withania somnifera–Based Indian Traditional Formulation (Amukkara Choornam), Against Chikungunya Virus–Induced Morbidity and Arthralgia. J. Evid. Based Integr. Med. 2018, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-H.; Wu, K.-L.; Zhang, X.; Deng, S.-Q.; Peng, B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020, 18, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Symptomatic Protective Action of Glycyrrhizin (Licorice) in COVID-19 Infection? Front. Immunol. 2020, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-J.; Zheng, X.-W.; Feng, T.; Huang, J.; Chen, J.; Wu, Y.-Y.; Zhou, L.-M.; Tu, W.-W.; Li, H. Andrographolide exerted its antimicrobial effects by upregulation of human β-defensin-2 induced through p38 MAPK and NF-κB pathway in human lung epithelial cells. Can. J. Physiol. Pharmacol. 2012, 90, 647–653. [Google Scholar] [CrossRef]
- Xiong, W.-B.; Shao, Z.-J.; Xiong, Y.; Chen, J.; Sun, Y.; Zhu, L.; Zhou, L.-M. Dehydroandrographolide enhances innate immunity of intestinal tract through up-regulation the expression of hBD-2. Daru J. Pharm. Sci. 2015, 23, 37. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Wang, J. Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2016, 13, 1827–1832. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Shim, H.-J.; Shin, H.-M.; Lee, Y.J.; Nam, H.; Kim, S.Y.; Youn, H.-S. Andrographolide suppresses TRIF-dependent signaling of toll-like receptors by targeting TBK1. Int. Immunopharmacol. 2018, 57, 172–180. [Google Scholar] [CrossRef]
- Han, S.B.; Yoon, Y.D.; Ahn, H.J.; Lee, H.S.; Lee, C.W.; Yoon, W.K.; Park, S.K.; Kim, H.M. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int. Immunopharmacol. 2003, 3, 1301–1312. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.H.; Shin, Y.S.; Kang, H.; Cho, H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharmacal Res. 2017, 40, 623–630. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, S.H.; Kim, J.Y.; Lee, W.S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. J. Thorac. Dis. 2019, 11, 1287–1302. [Google Scholar] [CrossRef]
- Peng, L.N.; Li, L.; Qiu, Y.F.; Miao, J.H.; Gao, X.Q.; Zhou, Y.; Shi, Z.X.; Xu, Y.L.; Shao, D.H.; Wei, J.C.; et al. Glycyrrhetinic acid extracted from Glycyrrhiza uralensis Fisch. induces the expression of Toll-like receptor 4 in Ana-1 murine macrophages. J. Asian Nat. Prod. Res. 2011, 13, 942–950. [Google Scholar] [CrossRef]
- Schröfelbauer, B.; Raffetseder, J.; Hauner, M.; Wolkerstorfer, A.; Ernst, W.; Szolar Oliver, H.J. Glycyrrhizin, the main active compound in liquorice, attenuates pro-inflammatory responses by interfering with membrane-dependent receptor signalling. Biochem. J. 2009, 421, 473–482. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish. Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef]
- Ahn, H.; Han, B.-C.; Kim, J.; Kang, S.G.; Kim, P.-H.; Jang, K.H.; So, S.H.; Lee, S.-H.; Lee, G.-S. Nonsaponin fraction of Korean Red Ginseng attenuates cytokine production via inhibition of TLR4 expression. J. Ginseng Res. 2019, 43, 291–299. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Choi, I.S.; Shim, J.Y.; Yun, E.K.; Yun, Y.S.; Jeong, G.; Song, J.Y. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur. J. Immunol. 2006, 36, 37–45. [Google Scholar] [CrossRef]
- Kim, T.-W.; Joh, E.-H.; Kim, B.; Kim, D.-H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int. Immunopharmacol. 2012, 12, 110–116. [Google Scholar] [CrossRef]
- Nakaya, T.A.; Kita, M.; Kuriyama, H.; Iwakura, Y.; Imanishi, J. Panax ginseng Induces Production of Proinflammatory Cytokines via Toll-like Receptor. J. Interferon Cytokine Res. 2004, 24, 93–100. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Luong, T.T.; Lee, S.Y.; Kim, G.L.; Kwon, H.; Lee, H.-G.; Park, C.-K.; Rhee, D.-K. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine 2015, 22, 1055–1061. [Google Scholar] [CrossRef]
- Paik, S.; Choe, J.H.; Choi, G.-E.; Kim, J.-E.; Kim, J.-M.; Song, G.Y.; Jo, E.-K. Rg6, a rare ginsenoside, inhibits systemic inflammation through the induction of interleukin-10 and microRNA-146a. Sci. Rep. 2019, 9, 4342. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Ganju, L.; Chanda, S.; Karan, D.; Sawhney, R.C. Aqueous extract of Rhodiola imbricata rhizome stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in vitro. Immunobiology 2009, 214, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Ganju, L.; Singh, S.B. Anti-cellular and immunomodulatory potential of aqueous extract of Rhodiola imbricata rhizome. Immunopharmacol. Immunotoxicol. 2012, 34, 513–518. [Google Scholar] [CrossRef]
- Shan, Y.; Jiang, B.; Yu, J.; Wang, J.; Wang, X.; Li, H.; Wang, C.; Chen, J.; Sun, J. Protective Effect of Schisandra chinensis Polysaccharides Against the Immunological Liver Injury in Mice Based on Nrf2/ARE and TLR4/NF-κB Signaling Pathway. J. Med. Food 2019, 22, 885–895. [Google Scholar] [CrossRef]
- Sun, K.; Huang, R.; Yan, L.; Li, D.-T.; Liu, Y.-Y.; Wei, X.-H.; Cui, Y.-C.; Pan, C.-S.; Fan, J.-Y.; Wang, X.; et al. Schisandrin Attenuates Lipopolysaccharide-Induced Lung Injury by Regulating TLR-4 and Akt/FoxO1 Signaling Pathways. Front. Physiol. 2018, 9, 1104. [Google Scholar] [CrossRef]
- Zhao, T.; Feng, Y.; Li, J.; Mao, R.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Chen, Y.; Yang, L.; et al. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int. J. Biol. Macromol. 2014, 65, 33–40. [Google Scholar] [CrossRef]
- Maitra, R.; Porter, M.A.; Huang, S.; Gilmour, B.P. Inhibition of NFκB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J. Inflamm. 2009, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.-W.; Koh, E.-J.; Lee, S.-M. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J. Pineal Res. 2011, 50, 403–411. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef]
- Kowalewska, M.; Herman, A.P.; Szczepkowska, A.; Skipor, J. The effect of melatonin from slow-release implants on basic and TLR-4-mediated gene expression of inflammatory cytokines and their receptors in the choroid plexus in ewes. Res. Vet. Sci. 2017, 113, 50–55. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, G.; Ai, L.; Shi, J.; Zhang, J.; Chen, Y.-X. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 2018, 8, 15579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.; Davtyan, T.; Gukassyan, N.; Gukasova, G.; Mamikonyan, G.; Gabrielian, E.; Wikman, G. Effect of Andrographolide and Kan Jang—Fixed combination of extract SHA-10 and extract SHE-3—On proliferation of human lymphocytes, production of cytokines and immune activation markers in the whole blood cells culture. Phytomedicine 2002, 9, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Zykov, M.P.; Protasova, S.F. Prospects of immunostimulating vaccination against influenza including the use of Eleutherococcus and other preparations of plant origin. In New Data on Eleutherococcus, Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow, 1984; Far East Academy of Sciences of the USSR: Vladivostok, USSR, 1986; pp. 118–122. [Google Scholar]
- Bohn, B.; Nebe, C.T.; Birr, C. Flow-cytometric studies with eleutherococcus senticosus extract as an immunomodulatory agent. Arzneim. Forsch. 1987, 37, 1193–1196. [Google Scholar]
- Bohn, B.; Nebe, C.T.; Birr, C. Immunopharmacological effects of eleutherococcus senticosus extract as determined by quantitative flow cytometry. Int. J. Immunopharmacol. 1988, 10, 67. [Google Scholar] [CrossRef]
- Kupin, V.I.; Polevaya, E.S.; Sorokin, A.M. Increased immunologic reactivity of lymphocytes in oncologic patients treated with Eleutherococcus extract. In New Data on Eleutherococcus, Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow, 1984; Far East Academy of Sciences of the USSR: Vladivostok, USSR, 1986; pp. 216–220. [Google Scholar]
- Wacker, A. Über die Interferon induzierende und immunstimulierende Wirkung von Eleutherococcus. Erfahrungsheilkunde 1983, 32, 339–343. [Google Scholar]
- Wacker, A.; Eichler, A.; Lodemann, E. The molecular mechanism of virus inhibition by Eleutherococcus. In New Data on Eleutherococcus, Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow, 1984; Far East Academy of Sciences of the USSR: Vladivostok, USSR, 1986; pp. 13–15. [Google Scholar]
- Wacker, A.; Eilmes, H.G. Virushemmung mit Eleutherokokk Fluid-Extrakt. Erfahrungsheilkunde 1978, 27, 346–351. [Google Scholar]
- Kour, K.; Pandey, A.; Suri, K.A.; Satti, N.K.; Gupta, K.K.; Bani, S. Restoration of stress-induced altered T cell function and corresponding cytokines patterns by Withanolide A. Int. Immunopharmacol. 2009, 9, 1137–1144. [Google Scholar] [CrossRef]
- Malik, F.; Singh, J.; Khajuria, A.; Suri, K.A.; Satti, N.K.; Singh, S.; Kaul, M.K.; Kumar, A.; Bhatia, A.; Qazi, G.N. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007, 80, 1525–1538. [Google Scholar] [CrossRef]
- Khan, B.; Ahmad, S.F.; Bani, S.; Kaul, A.; Suri, K.A.; Satti, N.K.; Athar, M.; Qazi, G.N. Augmentation and proliferation of T lymphocytes and Th-1 cytokines by Withania somnifera in stressed mice. Int. Immunopharmacol. 2006, 6, 1394–1403. [Google Scholar] [CrossRef]
- Khan, S.; Malik, F.; Suri, K.A.; Singh, J. Molecular insight into the immune up-regulatory properties of the leaf extract of Ashwagandha and identification of Th1 immunostimulatory chemical entity. Vaccine 2009, 27, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Kuo, Y.-H.; Hsieh, S.-L.; Lin, B.-F. Inhibitory effects of ethyl acetate extract of Andrographis paniculata on NF-κB trans-activation activity and LPS-induced acute inflammation in mice. Evid. Based Complementary Altern. Med. 2011, 2011, 254531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.; Hambartsumyan, M.; Panosyan, L.; Abrahamyan, H.; Mamikonyan, G.; Gabrielyan, E.; Amaryan, G.; Astvatsatryan, V.; Wikman, G. Plasma nitric oxide level in familial Mediterranean fever and its modulations by Immuno-Guard. Nitric Oxide 2003, 9, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Schmiech, M.; El Gaafary, M.; Zhang, X.; Syrovets, T.; Simmet, T. A comparative study on root and bark extracts of Eleutherococcus senticosus and their effects on human macrophages. Phytomedicine 2020, 68, 153181. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G. Adaptogens: Tonic Herbs for Fatigue and Stress. Altern. Complementary Ther. 2003, 9, 327–331. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wang, Y.; Mu, P.; Chen, C.; Huang, P.; Liu, D. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. 2019, 19, 3633–3641. [Google Scholar] [CrossRef]
- Iqbal, H.; Rhee, D.-K. Ginseng alleviates microbial infections of the respiratory tract: A review. J. Ginseng Res. 2020, 44, 194–204. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Ju, J.; Wang, Y.; Liu, Z.; Zhao, X.; Yan, Y.; Yan, S.; Luo, X.; Fang, Y. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am. J. Transl. Res. 2019, 11, 199–209. [Google Scholar]
- Vanden Berghe, W.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin, A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- Sánchez-López, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramírez-Jirano, L.J.; Velázquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef]
- Schmolz, M.W.; Sacher, F.; Aicher, B. The synthesis of Rantes, G-CSF, IL-4, IL-5, IL-6, IL-12 and IL-13 in human whole-blood cultures is modulated by an extract from Eleutherococcus senticosus L. roots. Phytother. Res. 2001, 15, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.P.; Das, B.K.; Singh, R.; Tyagi, S. Effect of Withania somnifer on CD38 expression on CD8+ T lymphocytes among patients of HIV infection. Clin. Immunol. 2019, 203, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Yarla, N.S.; Zameer, F.; Nagendra Prasad, M.N.; Santosh, M.S.; More, S.S.; Rao, D.G.; Dhananjaya, B.L. Inhibition of Group IIA Secretory Phospholipase A2 and its Inflammatory Reactions in Mice by Ethanolic Extract of Andrographis paniculata, a Well-known Medicinal Food. Pharmacogn. Res. 2016, 8, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, H.; Wang, Y.; Zheng, H.; Yu, W.; Chai, H.; Zhang, J.; Falck, J.R.; Guo, A.M.; Yue, J.; et al. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer. Toxicol. Appl. Pharmacol. 2013, 272, 37–48. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Bao, Y.; Li, T.; Chang, X.; Yang, G.; Meng, X. Multipathway Integrated Adjustment Mechanism of Glycyrrhiza Triterpenes Curing Gastric Ulcer in Rats. Pharm. Mag. 2017, 13, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Li, X.; Wu, J.; Liang, Z.; Deng, F.; Xie, W.; Zhu, M.; Zhu, J.; Zhu, W.; Geng, S.; et al. Anti-inflammatory Activity of Magnesium Isoglycyrrhizinate Through Inhibition of Phospholipase A2/Arachidonic Acid Pathway. Inflammation 2015, 38, 1639–1648. [Google Scholar] [CrossRef]
- Cha, T.W.; Kim, M.; Kim, M.; Chae, J.S.; Lee, J.H. Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA 2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertens. Res. 2016, 39, 449–456. [Google Scholar] [CrossRef]
- Irfan, M.; Kim, M.; Rhee, M.H. Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. J. Ginseng Res. 2020, 44, 24–32. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ro, J.Y.; Lee, C.H. 20(S)-Protopanaxatriol inhibits release of inflammatory mediators in immunoglobulin E-mediated mast cell activation. J. Ginseng Res. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kwon, H.-W.; Rhee, M.H.; Park, H.-J. Inhibitory effects of thromboxane A2 generation by ginsenoside Ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release. J. Ginseng Res. 2019, 43, 236–241. [Google Scholar] [CrossRef]
- Bawa, A.S.; Khanum, F. Anti-inflammatory activity of Rhodiola rosea—“A second-generation adaptogen”. Phytother. Res. 2009, 23, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, Y.; Mizoguchi, Y.; Morisawa, S.; Takeda, S.; Aburada, M.; Hosoya, E. Effect of Gomisin A (TJN-101) on the Arachidonic Acid Cascade in Macrophages. Jpn. J. Pharmacol. 1990, 52, 331–336. [Google Scholar] [CrossRef]
- Lizano, S.; Domont, G.; Perales, J. Natural phospholipase A2 myotoxin inhibitor proteins from snakes, mammals and plants. Toxicon 2003, 42, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Machiah, D.K.; Gowda, T.V. Purification of a post-synaptic neurotoxic phospholipase A2 from Naja naja venom and its inhibition by a glycoprotein from Withania somnifera. Biochimie 2006, 88, 701–710. [Google Scholar] [CrossRef]
- Madhusudan, M.; Zameer, F.; Naidu, A.; Dhananjaya, B.L.; Hegdekatte, R. Evaluating the inhibitory potential of Withania somnifera on platelet aggregation and inflammation enzymes: An in vitro and in silico study. Pharm. Biol. 2016, 54, 1936–1941. [Google Scholar] [CrossRef] [Green Version]
- Chao, W.-W.; Kuo, Y.-H.; Li, W.-C.; Lin, B.-F. The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J. Ethnopharmacol. 2009, 122, 68–75. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.-J.; Efferth, T. Effects of anti-inflammatory and adaptogenic herbal extracts on gene expression of eicosanoids signaling pathways in isolated brain cells. Phytomedicine 2019, 60, 152881. [Google Scholar] [CrossRef] [PubMed]
- Amroyan, E.; Gabrielian, E.; Panossian, A.; Wikman, G.; Wagner, H. Inhibitory effect of andrographolide from Andrographis paniculata on PAF-induced platelet aggregation. Phytomedicine 1999, 6, 27–31. [Google Scholar] [CrossRef]
- Burgos, R.A.; Hidalgo, M.A.; Monsalve, J.; LaBranche, T.P.; Eyre, P.; Hancke, J.L. 14-deoxyandrographolide as a platelet activating factor antagonist in bovine neutrophils. Planta Med. 2005, 71, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Kim, D.S.; Oh, S.R.; Lee, I.S.; Lee, J.J.; Park, J.D.; Kim, S.I.; Lee, H.-K. Platelet Activating Factor Antagonist Activity of Ginsenosides. Biol. Pharm. Bull. 1998, 21, 79–80. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.-M.; Kuo, S.-C.; Ko, F.-N.; Lee, J.-C.; Lee, L.-G.; Chen, S.-C.; Huang, T.-F. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 315–320. [Google Scholar] [CrossRef]
- Jung, K.Y.; Lee, I.S.; Oh, S.R.; Kim, D.S.; Lee, H.K. Lignans with platelet activating factor antagonist activity from Schisandra chinensis (Turcz.) Baill. Phytomedicine 1997, 4, 229–231. [Google Scholar] [CrossRef]
- Lee, I.S.; Jung, K.Y.; Oh, S.R.; Park, S.H.; Ahn, K.S.; Lee, H.-K. Structure-Activity Relationships of Lignans from Schisandra chinensis as Platelet Activating Factor Antagonists. Biol. Pharm. Bull. 1999, 22, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Chiou, W.-F.; Chen, C.-F.; Lin, J.-J. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 2000, 129, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Panossian, A.; Hambardzumyan, M.; Hovhanissyan, A.; Wikman, G. The Adaptogens Rhodiola and Schizandra Modify the Response to Immobilization Stress in Rabbits by Suppressing the Increase of Phosphorylated Stress-activated Protein Kinase, Nitric Oxide and Cortisol. Drug Target. Insights 2007, 2. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Chen, S.-R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef]
- Fei, X.J.; Zhu, L.L.; Xia, L.M.; Peng, W.B.; Wang, Q. Acanthopanax senticosus attenuates inflammation in lipopolysaccharide-induced acute lung injury by inhibiting the NF-κB pathway. Genet. Mol. Res. 2014, 13, 10537–10544. [Google Scholar] [CrossRef]
- Han, J.; Liu, L.; Yu, N.; Chen, J.; Liu, B.; Yang, D.; Shen, G. Polysaccharides from Acanthopanax senticosus enhances intestinal integrity through inhibiting TLR4/NF-κB signaling pathways in lipopolysaccharide-challenged mice. Anim. Sci. J. 2016, 87, 1011–1018. [Google Scholar] [CrossRef]
- Kim, J.-A.; Kim, D.-K.; Jin, T.; Kang, O.-H.; Choi, Y.-A.; Choi, S.-C.; Kim, T.-H.; Nah, Y.-H.; Choi, S.-J.; Kim, Y.-H.; et al. Acanthoic acid inhibits IL-8 production via MAPKs and NF-κB in a TNF-α-stimulated human intestinal epithelial cell line. Clin. Chim. Acta 2004, 342, 193–202. [Google Scholar] [CrossRef]
- Lin, Q.-Y.; Jin, L.-J.; Cao, Z.-H.; Li, H.-Q.; Xu, Y.-P. Protective effect of Acanthopanax senticosus extract against endotoxic shock in mice. J. Ethnopharmacol. 2008, 118, 495–502. [Google Scholar] [CrossRef]
- Lin, Q.-Y.; Jin, L.-J.; Cao, Z.-H.; Xu, Y.-P. Inhibition of inducible nitric oxide synthase by Acanthopanax senticosus extract in RAW264.7 macrophages. J. Ethnopharmacol. 2008, 118, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, S.Y.; Kim, E.K.; Ryu, E.Y.; Kim, Y.H.; Park, G.; Lee, S.J. Acanthopanax senticosus has a heme oxygenase-1 signaling-dependent effect on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2012, 142, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, X.; Wei, R.; Wang, C.; Xue, X.; Mao, L. Protective effects of Acanthopanax vs. Ulinastatin against severe acute pancreatitis-induced brain injury in rats. Int. Immunopharmacol. 2015, 24, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Shimosaka, S.; Sasaki, H.; Matsumura, T.; Tukiyama, T.; Tokiwa, T. (+)-Syringaresinol-di-O-β-d-glucoside, a phenolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SW982 human synovial sarcoma cells by inhibiting activating protein-1 and/or nuclear factor-κB activities. Toxicol. Vitr. 2007, 21, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Z.; Sheng, L.; Wu, H. Protective effects of syringin against lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2017, 209, 252–257. [Google Scholar] [CrossRef]
- Kang, B.; Kim, C.Y.; Hwang, J.; Sun, S.; Yang, H.; Suh, H.J.; Choi, H.-S. Red ginseng extract regulates differentiation of monocytes to macrophage and inflammatory signalings in human monocytes. Food Sci. Biotechnol. 2019, 28, 1819–1828. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Choi, B.M.; Seo, E.A.; Kim, D.H.; Shin, M.K.; Kim, J.D.; Kim, J.B.; Chung, H.T. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-κB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004, 205, 23–29. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Lee, J.-Y.; Choi, K.-J.; Ko, S.-R. Effects of Selected Ginsenosides on Phorbol Ester-Induced Expression of Cyclooxygenase-2 and Activation of NF-κB and ERK1/2 in Mouse Skin. Ann. N. Y. Acad. Sci. 2002, 973, 396–401. [Google Scholar] [CrossRef]
- Surh, Y.J.; Na, H.K.; Lee, J.Y.; Keum, Y.S. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J. Korean Med. Sci 2001, 16, S38–S41. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Han, S.S.; Chun, K.-S.; Park, K.-K.; Park, J.-H.; Lee, S.K.; Surh, Y.-J. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-κB activation and tumor promotion. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 523–524, 75–85. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Dalia, P.; Corsi, L. Rhodiola rosea L. modulates inflammatory processes in a CRH-activated BV2 cell model. Phytomedicine 2020, 68, 153143. [Google Scholar] [CrossRef]
- Hu, R.; Wang, M.-Q.; Ni, S.-H.; Wang, M.; Liu, L.-Y.; You, H.-Y.; Wu, X.-H.; Wang, Y.-J.; Lu, L.; Wei, L.-B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020, 867, 172797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Fan, L.-Y.; Yuan, M.-D.; Xing, M.-Y. Salidroside Inhibits Lipopolysaccharide-ethanol-induced Activation of Proinflammatory Macrophages via Notch Signaling Pathway. Curr. Med. Sci. 2019, 39, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, L.; Mao, J.; He, H.; Liu, J.; Cai, X.; Lin, H.; Wu, T. Salidroside protects against bleomycin-induced pulmonary fibrosis: Activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones 2016, 21, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.; Yao, D.; Zhang, K.; Han, S.; Liu, D.; Wang, H.; Liu, X.; Li, G.; Huang, J.; Wang, J. Protective effects of Rosavin on bleomycin-induced pulmonary fibrosis via suppressing fibrotic and inflammatory signaling pathways in mice. Biomed. Pharmacother. 2019, 115, 108870. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, Y.; Guo, R.; Zang, W. Salidroside Protects Against Advanced Glycation End Products-Induced Vascular Endothelial Dysfunction. Med. Sci. Monit. 2018, 24, 2420–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lai, W.; Ying, X.; Xu, L.; Chu, K.; Brown, J.; Chen, L.; Hong, G. Salidroside Reduces Inflammation and Brain Injury After Permanent Middle Cerebral Artery Occlusion in Rats by Regulating PI3K/PKB/Nrf2/NFκB Signaling Rather than Complement C3 Activity. Inflammation 2019, 42, 1830–1842. [Google Scholar] [CrossRef]
- Ci, X.; Ren, R.; Xu, K.; Li, H.; Yu, Q.; Song, Y.; Wang, D.; Li, R.; Deng, X. Schisantherin A Exhibits Anti-inflammatory Properties by Down-Regulating NF-κB and MAPK Signaling Pathways in Lipopolysaccharide-Treated RAW 264.7 Cells. Inflammation 2010, 33, 126–136. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.-J.; Choi, E.O.; Leem, S.-H.; Kim, G.-Y.; Moon, S.-K.; Chang, Y.-C.; Yun, S.-J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Cheng, B.C.-Y.; Zhao, H.; Fu, X.-Q.; Xie, R.; Zhang, S.-F.; Pan, S.-Y.; Zhang, Y. Schisandra Chinensis Lignans Suppresses the Production of Inflammatory Mediators Regulated by NF-κB, AP-1, and IRF3 in Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules 2018, 23, 3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, J.; Ma, C.; Xu, K.; Xu, L.; He, Y.; Moqbel, S.A.A.; Hu, P.; Jiang, L.; Chen, W.; Bao, J.; et al. Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-κB and MAPK signal pathways. Drug Des. Devel. Ther. 2018, 12, 1195–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.-J.; Zeng, K.-W.; Chen, J.-F.; Li, Y.; Song, X.-M.; Tu, P.-F.; Wang, X.-M. Extract of Fructus Schisandrae chinensis Inhibits Neuroinflammation Mediator Production from Microglia via NF-κ B and MAPK Pathways. Chin. J. Integr. Med. 2019, 25, 131–138. [Google Scholar] [CrossRef]
- Heyninck, K.; Lahtela-Kakkonen, M.; Van der Veken, P.; Haegeman, G.; Vanden Berghe, W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef]
- Mulabagal, V.; Subbaraju, G.V.; Rao, C.V.; Sivaramakrishna, C.; DeWitt, D.L.; Holmes, D.; Sung, B.; Aggarwal, B.B.; Tsay, H.-S.; Nair, M.G. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother. Res. 2009, 23, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kwon, T.K. Withaferin A inhibits tumor necrosis factor-α-induced expression of cell adhesion molecules by inactivation of Akt and NF-κB in human pulmonary epithelial cells. Int. Immunopharmacol. 2009, 9, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Aggarwal, A.; Maurya, R.; Naik, S. Withania somnifera inhibits NF-κB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother. Res. 2007, 21, 905–913. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Adeoye, B.O.; Asenuga, E.R.; Oyagbemi, A.A.; Omobowale, T.O.; Adedapo, A.A. The Protective Effect of the Ethanol Leaf Extract of Andrographis Paniculata on Cisplatin-Induced Acute Kidney Injury in Rats Through nrf2/KIM-1 Signalling Pathway. Drug Res. 2018, 68, 23–32. [Google Scholar] [CrossRef]
- Lee, J.-C.; Tseng, C.-K.; Young, K.-C.; Sun, H.-Y.; Wang, S.-W.; Chen, W.-C.; Lin, C.-K.; Wu, Y.-H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. [Google Scholar] [CrossRef]
- Lin, H.-C.; Su, S.-L.; Lu, C.-Y.; Lin, A.-H.; Lin, W.-C.; Liu, C.-S.; Yang, Y.-C.; Wang, H.-M.; Lii, C.-K.; Chen, H.-W. Andrographolide inhibits hypoxia-induced HIF-1α-driven endothelin 1 secretion by activating Nrf2/HO-1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Environ. Toxicol. 2017, 32, 918–930. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Yang, Y.-C.; Li, C.-C.; Liu, K.-L.; Lii, C.-K.; Chen, H.-W. Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells. Biochem. Pharmacol. 2014, 91, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.-W.; Yang, S.-X.; Pan, Z.-Z.; Zheng, B.; Wang, J.-Z.; Lu, G.-R.; Xue, Z.-X.; Xu, C.-L. Andrographolide ameliorates d-galactosamine/lipopolysaccharide-induced acute liver injury by activating Nrf2 signaling pathway. Oncotarget 2017, 8, 41202–41210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.Y.; Pyo, E.; An, J.-P.; Kim, J.; Sung, S.H.; Oh, W.K. Andrographolide Activates Keap1/Nrf2/ARE/HO-1 Pathway in HT22 Cells and Suppresses Microglial Activation by Aβ42 through Nrf2-Related Inflammatory Response. Mediat. Inflamm. 2017, 2017, 5906189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Wong, W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017, 139, 71–81. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, M.G.K.; Wong, P.T.H.; Herr, D.R.; Lai, M.K.P. Andrographolide induces Nrf2 and heme oxygenase 1 in astrocytes by activating p38 MAPK and ERK. J. Neuroinflammation 2016, 13, 251. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hai, C.X.; Liang, X.; Yu, S.X.; Zhang, W.; Li, Y.L. The protective effects of Acanthopanax senticosus Harms aqueous extracts against oxidative stress: Role of Nrf2 and antioxidant enzymes. J. Ethnopharmacol. 2010, 127, 424–432. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, Z.; Zhou, X.; He, W.B.; Chen, C.; Luo, P.; Liu, D.D.; Ai, Q.D.; Gong, H.F.; Wang, Z.Z.; et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol. Sin. 2019, 40, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-P.; Gao, Y.; Chu, S.-F.; Zhang, Z.; Xia, C.-Y.; Mou, Z.; Song, X.-Y.; He, W.-B.; Guo, X.-F.; Chen, N.-H. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis. Acta Pharmacol. Sin. 2014, 35, 1031–1044. [Google Scholar] [CrossRef] [Green Version]
- Saw, C.L.L.; Yang, A.Y.; Cheng, D.C.; Boyanapalli, S.S.S.; Su, Z.-Y.; Khor, T.O.; Gao, S.; Wang, J.; Jiang, Z.-H.; Kong, A.-N.T. Pharmacodynamics of Ginsenosides: Antioxidant Activities, Activation of Nrf2, and Potential Synergistic Effects of Combinations. Chem. Res. Toxicol. 2012, 25, 1574–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaukat, A.; Yang, C.; Yang, Y.; Guo, Y.-F.; Jiang, K.; Guo, S.; Liu, J.; Zhang, T.; Zhao, G.; Ma, X.; et al. Ginsenoside Rb 1: A novel therapeutic agent in Staphylococcus aureus-induced Acute Lung Injury with special reference to Oxidative stress and Apoptosis. Microb. Pathog. 2020, 143, 104109. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xiao, Q.; Lin, Y.-H.; Zheng, Z.-Z.; He, Z.-D.; Hu, J.; Chen, L.-D. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen Res. 2015, 10, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Li, T.; Wu, L.; Fang, Y.; Feng, Y.; Zhang, L.; Chen, J.; Wang, X. Salidroside Protects Dopaminergic Neurons by Preserving Complex I Activity via DJ-1/Nrf2-Mediated Antioxidant Pathway. Parkinsons Dis. 2019, 2019, 6073496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhang, Y.-J.; Liu, W.-W.; Shi, A.-W.; Gu, N. Salidroside Suppresses HUVECs Cell Injury Induced by Oxidative Stress through Activating the Nrf2 Signaling Pathway. Molecules 2016, 21, 1033. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Yu, Z.; Jing, S.; Jiang, W.; Liu, C.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Protective effect of Anwulignan against D-galactose-induced hepatic injury through activating p38 MAPK-Nrf2-HO-1 pathway in mice. Clin. Interv. Aging 2018, 13, 1859–1869. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Geng, Q.; Huang, H.; Yao, H.; Du, T.; Chen, L.; Wu, Z.; Miao, X.; Shi, P. Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules 2019, 24, 1090. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jing, S.; Lin, H.; Sun, W.; Jiang, W.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice. Food Funct. 2019, 10, 7755–7766. [Google Scholar] [CrossRef]
- Heyninck, K.; Sabbe, L.; Chirumamilla, C.S.; Szarc vel Szic, K.; Vander Veken, P.; Lemmens, K.J.A.; Lahtela-Kakkonen, M.; Naulaerts, S.; Op de Beeck, K.; Laukens, K.; et al. Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem. Pharmacol. 2016, 109, 48–61. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Reuland, D.J.; Khademi, S.; Castle, C.J.; Irwin, D.C.; McCord, J.M.; Miller, B.F.; Hamilton, K.L. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic. Biol. Med. 2013, 56, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-J.; Chung, Y.H.; Le, H.-L.T.; Jeong, J.H.; Dang, D.-K.; Nam, Y.; Wie, M.B.; Nah, S.-Y.; Nabeshima, Y.-I.; Nabeshima, T.; et al. Melatonin Attenuates Memory Impairment Induced by Klotho Gene Deficiency Via Interactive Signaling Between MT2 Receptor, ERK, and Nrf2-Related Antioxidant Potential. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carota, G.; Raffaele, M.; Sorrenti, V.; Salerno, L.; Pittalà, V.; Intagliata, S. Ginseng and heme oxygenase-1: The link between an old herb and a new protective system. Fitoterapia 2019, 139, 104370. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Kaur, P.; Panossian, A.; Wikman, K.G. Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts. Phytomedicine 2013, 20, 1323–1329. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine 2009, 16, 617–622. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens Stimulate Neuropeptide Y and Hsp72 Expression and Release in Neuroglia Cells. Front. Neurosci. 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Zhao, Y.; Xu, L.; Gao, L.; Su, Y.; Lin, N.; Pu, J. The nuclear melatonin receptor RORα is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 2016, 60, 313–326. [Google Scholar] [CrossRef]
- Thakur, A.; Dey, A.; Chatterjee, S.; Kumar, V. Reverse Ayurvedic Pharmacology of Ashwagandha as an Adaptogenic Anti-Diabetic Plant: A Pilot Study. Curr. Tradit. Med. 2015, 1, 51–61. [Google Scholar] [CrossRef]
- Thakur, A.K.; Chatterjee, S.S.; Kumar, V. Adaptogenic potential of andrographolide: An active principle of the king of bitters (Andrographis paniculata). J. Tradit. Complementary Med. 2015, 5, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Makanjuola, V.O.; Arora, R.; Singh, B.; Arora, S. Immunopotentiating significance of conventionally used plant adaptogens as modulators in biochemical and molecular signalling pathways in cell mediated processes. Biomed. Pharmacother. 2017, 95, 1815–1829. [Google Scholar] [CrossRef]
- EMA. Final Assessment Report on Glycyrrhiza Glabra L. and/or Glycyrrhiza Inflata Bat. and/or Glycyrrhiza Uralensis Fisch., Radix; Committee on Herbal Medicinal Products (HMPC). European Medicines Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
- EMA. Final Assessment Report on Panax Ginseng C.A. Meyer, Radix; Committee on Herbal Medicinal Products (HMPC). European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- EMA. Final Assessment Report on Rhodiola Rosea; Committee on Herbal Medicinal Products (HMPC). European Medicines Agency: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Błasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of Schisandra chinensis (Turcz.) Baill. in Human Health and Nutrition: A Review of Current Knowledge and Therapeutic Perspectives. Nutrients 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Szopa, A. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Kalra, R.; Kaushik, N. Withania somnifera (Linn.) Dunal: A review of chemical and pharmacological diversity. Phytochem. Rev. 2017, 16, 953–987. [Google Scholar] [CrossRef]
- Tripathi, N.; Shrivastava, D.; Ahmad Mir, B.; Kumar, S.; Govil, S.; Vahedi, M.; Bisen, P.S. Metabolomic and biotechnological approaches to determine therapeutic potential of Withania somnifera (L.) Dunal: A review. Phytomedicine 2018, 50, 127–136. [Google Scholar] [CrossRef]
- Reyes, B.A.S.; Bautista, N.D.; Tanquilut, N.C.; Anunciado, R.V.; Leung, A.B.; Sanchez, G.C.; Magtoto, R.L.; Castronuevo, P.; Tsukamura, H.; Maeda, K.I. Anti-diabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. J. Ethnopharmacol. 2006, 105, 196–200. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z. Inhibition of α-Glucosidase by Andrographis paniculata. Ethanol Extract in Rats. Pharm. Biol. 2006, 44, 600–606. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.K.-H. Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol. Sin. 2000, 21, 1157–1164. [Google Scholar] [PubMed]
- Zhang, X.F.; Tan, B.K.H. Antihyperglycaemic and anti-oxidant properties of andrographis paniculata in normal and diabetic rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 358–363. [Google Scholar] [CrossRef] [PubMed]
- EMA. Final Assessment Report on Eleutherococcus Senticosus (Rupr. et Maxim.) Maxim., Radix; Committee on Herbal Medicinal Products (HMPC). European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Akowuah, G.; Zhari, I.; Mariam, A.; Yam, M. Absorption of andrographolides from Andrographis paniculata and its effect on CCl4-induced oxidative stress in rats. Food Chem. Toxicol. 2009, 47, 2321–2326. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.L.; Wu, S.J.; Lee, S.C.; Ng, L.T. Antioxidant, antioedema and analgesic activities of Andrographis paniculata extracts and their active constituent andrographolide. Phytother. Res. 2009, 23, 958–964. [Google Scholar] [CrossRef]
- Verma, N.; Vinayak, M. Antioxidant action of Andrographis paniculata on lymphoma. Mol. Biol. Rep. 2008, 35, 535–540. [Google Scholar] [CrossRef]
- Sheeja, K.; Kuttan, G. Protective Effect of Andrographis paniculata and Andrographolide on Cyclophosphamide-Induced Urothelial Toxicity. Integr. Cancer Ther. 2006, 5, 244–251. [Google Scholar] [CrossRef]
- Saranya, P.; Geetha, A.; Selvamathy, S.N. A biochemical study on the gastroprotective effect of andrographolide in rats induced with gastric ulcer. Indian J. Pharm. Sci. 2011, 73, 550. [Google Scholar] [CrossRef] [Green Version]
- Wasman, S.Q.; Mahmood, A.A.; Chua, L.S.; Alshawsh, M.A.; Hamdan, S. Antioxidant and gastroprotective activities of Andrographis paniculata (Hempedu Bumi) in Sprague Dawley rats. Indian J. Exp. Biol. 2011, 49, 767–772. [Google Scholar]
- Chander, R.; Srivastava, V.; Tandon And, J.S.; Kapoor, N.K. Antihepatotoxic Activity of Diterpenes of Andrographis Paniculata (Kal-Megh) Against Plasmodium Berghei-Induced Hepatic Damage in Mastomys Natalensis. Int. J. Pharmacogn. 1995, 33, 135–138. [Google Scholar] [CrossRef]
- Koh, P.H.; Mokhtar, R.A.M.; Iqbal, M. Andrographis paniculata ameliorates carbon tetrachloride (CCl4)-dependent hepatic damage and toxicity: Diminution of oxidative stress. Redox Rep. 2011, 16, 134–143. [Google Scholar] [CrossRef]
- Nagalekshmi, R.; Menon, A.; Chandrasekharan, D.K.; Nair, C.K.K. Hepatoprotective activity of Andrographis Paniculata and Swertia Chirayita. Food Chem. Toxicol. 2011, 49, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Pramyothin, P.; Udomuksorn, W.; Poungshompoo, S.; Chaichantipyuth, C. Hepatoprotective effect of Andrographis paniculata and its constituent, andrographolide, on ethanol hepatotoxicity in rats. Asia Pac. J. Pharmacol. 1994, 9, 73–78. [Google Scholar]
- Wang, D.; Zhao, H. Prevention of atherosclerotic arterial stenosis and restenosis after angioplasty with Andrographis paniculata nees and fish oil. Experimental studies of effects and mechanisms. Chin. Med. J. 1994, 107, 464–470. [Google Scholar] [PubMed]
- Zhang, C.Y.; Tan, B.K.H. Mechanisms of cardiovascular activity of Andrographis paniculata in the anaesthetized rat. J. Ethnopharmacol. 1997, 56, 97–101. [Google Scholar] [CrossRef]
- Sheeja, K.; Guruvayoorappan, C.; Kuttan, G. Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int. Immunopharmacol. 2007, 7, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Kuttan, G. Modulation of Natural Killer Cell Activity, Antibody-Dependent Cellular Cytotoxicity, and Antibody-Dependent Complement-Mediated Cytotoxicity by Andrographolide in Normal and Ehrlich Ascites Carcinoma-Bearing Mice. Integr. Cancer Ther. 2007, 6, 66–73. [Google Scholar] [CrossRef]
- Sheeja, K.; Kuttan, G. Activation of Cytotoxic T Lymphocyte Responses and Attenuation of Tumor Growth in vivo by Andrographis paniculata Extract and Andrographolide. Immunopharmacol. Immunotoxicol. 2007, 29, 81–93. [Google Scholar] [CrossRef]
- Mandal, S.C.; Dhara, A.K.; Maiti, B.C. Studies on psychopharmacological activity ofAndrographis paniculata extract. Phytother. Res. 2001, 15, 253–256. [Google Scholar] [CrossRef]
- Thakur, A.K.; Soni, U.K.; Rai, G.; Chatterjee, S.S.; Kumar, V. Protective Effects of Andrographis paniculata Extract and Pure Andrographolide Against Chronic Stress-Triggered Pathologies in Rats. Cell. Mol. Neurobiol. 2014, 34, 1111–1121. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Gao, T.-T.; Wang, Y.; Wang, J.-L.; Guan, W.; Wang, Y.-J.; Wang, C.-N.; Liu, J.-F.; Jiang, B. Andrographolide Exerts Significant Antidepressant-Like Effects Involving the Hippocampal BDNF System in Mice. Int. J. Neuropsychopharmacol. 2019, 22, 585–600. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2007, 61, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Wu, R.-H.; Logue, M.; Blondel, C.; Lai, L.Y.W.; Stuart, B.; Flower, A.; Fei, Y.-T.; Moore, M.; Shepherd, J.; et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0181780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Caceres, D.; Hancke, J.; Burgos, R.; Sandberg, F.; Wikman, G. Use of visual analogue scale measurements (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomedicine 1999, 6, 217–223. [Google Scholar] [CrossRef]
- Melchior, J.; Palm, S.; Wikman, G. Controlled clinical study of standardized Andrographis paniculata extract in common cold—A pilot trial. Phytomedicine 1997, 3, 315–318. [Google Scholar] [CrossRef]
- Saxena, R.C.; Singh, R.; Kumar, P.; Yadav, S.C.; Negi, M.P.S.; Saxena, V.S.; Joshua, A.J.; Vijayabalaji, V.; Goudar, K.S.; Venkateshwarlu, K.; et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold™) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 2010, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Gagarin, I.A. Eleutherococcus in the Prophylaxis of the disease incidence in the Arctic. In Adaptation and Adaptogens, Proceedings of the 2nd Symposium, May 1975; Academy of Science of the USSR Far East Science Centre: Vladivostok, USSR, 1977; p. 128. [Google Scholar]
- Galanova, L.K. Eleutherococcus in preventive maintenance of a flu and relapses of hypertonic illness. In Adaptation and Adaptogens, Proceedings of the 2nd Symposium; Academy of Science of the USSR Far East Science Centre: Vladivostok, USSR, 1977; pp. 126–127. [Google Scholar]
- Schezin, A.K.; Zinkovich, V.I.; Matsuk, V.S. Tentative data on the mass Eleutherococcus prophylaxis of influenza at the main assembly line and metallurgic plant of the Volga Automobile Plant. Proceedings of 2nd All-Union Conference on Human Adaptation to Different Conditions, Novosibirsk, USSR; 1977; pp. 44–46. [Google Scholar]
- Shadrin, A.S.; Kustikova, Y.G.; Belogolovkina, N.A. Estimation of prophylactic and immunostimulating effects of Eleutherococcus and Schizandra chinensis preparations. In New Data on Eleutherococcus Proceedings of the II International Symposium on Eleutherococcus, Moscow, USSR, 1984; Far East Academy of Sciences of the USSR: Vladivostok, USSR, 1986; pp. 213–215. [Google Scholar]
- Barkan, A.; Gaĭduchenia, L.; Makarenko, I. Effect of Eleutherococcus on respiratory viral infectious morbidity in children in organized collectives. Pediatria 1980, 65–66. [Google Scholar]
- Sheparev, A.A.Z.; Kozlenko, I.Y. Effect of preventive administration of Eleutherococcus extract on health of children under school age. In New Data on Eleutherococcus Proceedings of the II International Symposium on Eleutherococcus, Moscow, USSR, 1984; Far East Academy of Sciences of the USSR: Vladivostok, USSR, 1986; pp. 201–203. [Google Scholar]
- Kwon, Y.J.; Son, D.H.; Chung, T.H.; Lee, Y.J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef]
- Scaglione, F.; Cattaneo, G.; Alessandria, M.; Cogo, R. Efficacy and safety of the standardised Ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold [corrected]. Drugs Exp. Clin. Res. 1996, 22, 65–72. [Google Scholar]
- Scaglione, F.; Weiser, K.; Alessandria, M. Effects of the Standardised Ginseng Extract G115® in Patients with Chronic Bronchitis. Clin. Drug Investig. 2001, 21, 41–45. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, J.H.; Oh, M.; Choi, K.-M.; Jeong, M.R.; Park, J.D.; Kwon, D.Y.; Ha, K.C.; Park, E.O.; Lee, N.; et al. Preventive Effect of Korean Red Ginseng for Acute Respiratory Illness: A Randomized and Double-Blind Clinical Trial. J. Korean Med. Sci. 2012, 27, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.-L.; Wu, T.-C.; Wang, Y.-T.; Wang, Y.-C.; Tsao, T.C.Y.; Wei, J.C.-C.; Chen, C.-Y.; Lin, I.F. Adjunctive Treatment with Rhodiola Crenulata in Patients with Chronic Obstructive Pulmonary Disease—A Randomized Placebo Controlled Double Blind Clinical Trial. PLoS ONE 2015, 10, e0128142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, W.; Xu, K.; Guo, Y.; Lin, S.; Xue, X.; Lu, G.; Li, N.; Liu, H.; Liu, W. Early use of Chinese drug rhodiola compound for patients with post-trauma and inflammation in prevention of ALI/ARDS. Zhonghua Wai Ke Za Zhi 1999, 37, 238–240. [Google Scholar] [PubMed]
- Lu, W.S.Z.; Cao, X.W.S.F. Early use of Chinese drug rhodiola compound for patients with thoracic operation inprevention of ALI. Med. J. Natl. Defending Forces Northwest China 2004, 2. [Google Scholar]
- Ahmed, M.; Henson, D.A.; Sanderson, M.C.; Nieman, D.C.; Zubeldia, J.M.; Shanely, R.A. Rhodiola rosea exerts antiviral activity in athletes following a competitive marathon race. Front. Nutr. 2015, 2, 24. [Google Scholar] [CrossRef]
- Yu, L.; Qin, Y.; Wang, Q.; Zhang, L.; Liu, Y.; Wang, T.; Huang, L.; Wu, L.; Xiong, H. The efficacy and safety of Chinese herbal medicine, Rhodiola formulation in treating ischemic heart disease: A systematic review and meta-analysis of randomized controlled trials. Complementary Ther. Med. 2014, 22, 814–825. [Google Scholar] [CrossRef]
- Lebedev, A.A. The effect of Schisandra seed tincture on morbidity rate among workers of Chirick shoe factory during the 1969 influenza epidemic. In Medicinal Products of the Far East; Brekhman, I.I., Fruentov, N.K., Eds.; Far East Branch of the USSR Academy of Science, Khabarovsk Medical Institute: Khabarovsk, USSR, 1970; pp. 115–119. [Google Scholar]
- Lebedev, A.A. Schizandra seed tincture and dibazole as means of non-specific prophylaxis of acute respiratory infections during the peak of influenza at the beginning of 1969. Med. Zhurnal Uzb. 1971, 6, 70–72. [Google Scholar]
- Pavlushchenko, E.V. Pneumonia in aged and old people in conditions of the monsoon climate of the southern Primorskij region. In New Data on Eleutherococcus and Other Adaptogens; Bulanov, A.E., Dardimov, I.V., Li, S.E., Eds.; Far East Branch of the USSR Academy of Science, Institute of Marine Biology: Vladivostok, USSR, 1981; pp. 119–122. [Google Scholar]
- Tandon, N.; Yadav, S.S. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol. 2020, 255, 112768. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Shah, B.; Shenoy, S.; Chauhan, S.; Lavekar, G.S.; Padhi, M.M. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int. J. Ayurveda Res. 2010, 1, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Caceres, D.; Hancke, J.; Burgos, R.; Wikman, G. Prevention of common colds with Andrographis paniculata dried extract. A pilot double blind trial. Phytomedicine 1997, 4, 101–104. [Google Scholar] [CrossRef]
- Gabrielian, E.S.; Shukarian, A.K.; Goukasova, G.I.; Chandanian, G.L.; Panossian, A.G.; Wikman, G.; Wagner, H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 2002, 9, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Kulichenko, L.L.; Kireyeva, L.V.; Malyshkina, E.N.; Wikman, G. A Randomized, Controlled Study of Kan Jang versus Amantadine in the Treatment of Influenza in Volgograd. J. Herb. Pharmacother. 2003, 3, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.; Spasov, A.A.; Ostrovskij, O.V.; Bulanov, A.E.; Wikman, G. Double-blind, placebo-controlled pilot and Phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 2000, 7, 341–350. [Google Scholar] [CrossRef]
- Spasov, A.A.; Ostrovskij, O.V.; Chernikov, M.V.; Wikman, G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang® and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. 2004, 18, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Anon. Periodic Safety Update Report for Kan Jang; Period Covered by this Report: From 23 November 2006 to 22 November 2009; Swedish Herbal Institute: Gothenburg, Sweden, 2010. [Google Scholar]
- Cook, D.N.; Kang, H.S.; Jetten, A.M. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl. Recept. Res. 2015, 2, 101185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jetten, A.M. Retinoid-Related Orphan Receptors (RORs): Critical Roles in Development, Immunity, Circadian Rhythm, and Cellular Metabolism. Nucl. Recept. Signal. 2009, 7, nrs.07003. [Google Scholar] [CrossRef] [Green Version]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin Antioxidative Defense: Therapeutical Implications for Aging and Neurodegenerative Processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [Green Version]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Arushanian, E.; Beĭer, E. Pineal hormone melatonin is an universal adaptogenic agent. Uspekhi Fiziol. Nauk 2012, 43, 82. [Google Scholar]
- Maestroni, G.J.M. Melatonin and the Immune System Therapeutic Potential in Cancer, Viral Diseases, and Immunodeficiency States. In The Pineal Gland and Cancer: Neuroimmunoendocrine Mechanisms in Malignancy; Bartsch, C., Bartsch, H., Blask, D.E., Cardinali, D.P., Hrushesky, W.J.M., Mecke, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 384–394. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current Status and Future Perspectives in Plant Science. Front. Plant. Sci. 2016, 6, 1230. [Google Scholar] [CrossRef] [Green Version]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant. Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Huo, Y.; Tan, D.-X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The potential of phytomelatonin as a nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef] [Green Version]
- Manchester, L.C.; Tan, D.-X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a Phytomelatonin-Rich Extract from Cultured Plants with Excellent Biochemical and Functional Properties as an Alternative to Synthetic Melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef] [PubMed]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liao, C.-L.; Chen, S.-J.; Shi, L.-G.; Lin, L.; Chen, Y.-W.; Cheng, C.-P.; Sytwu, H.-K.; Shang, S.-T.; Lin, G.-J. Melatonin possesses an anti-influenza potential through its immune modulatory effect. J. Funct. Foods 2019, 58, 189–198. [Google Scholar] [CrossRef]
- Huang, S.-H.; Cao, X.-J.; Liu, W.; Shi, X.-Y.; Wei, W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 2010, 48, 109–116. [Google Scholar] [CrossRef]
- Silvestri, M.; Rossi, G.A. Melatonin: Its possible role in the management of viral infections-a brief review. Ital. J. Pediatrics 2013, 39, 61. [Google Scholar] [CrossRef] [Green Version]
- Ben-Nathan, D.; Maestroni, G.; Lustig, S.; Conti, A. Protective effects of melatonin in mice infected with encephalitis viruses. Arch. Virol. 1995, 140, 223–230. [Google Scholar] [CrossRef]
- Bonilla, E.; Rodón, C.; Valero, N.; Pons, H.; Chacín-Bonilla, L.; Tamayo, J.G.; Rodríguez, Z.; Medina-Leendertz, S.; Añez, F. Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 207–210. [Google Scholar] [CrossRef]
- Bonilla, E.; Valero, N.; Chacín-Bonilla, L.; Medina-Leendertz, S. Melatonin and viral infections. J. Pineal Res. 2004, 36, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Valero-Fuenmayor, N.; Pons, H.; Chacin-Bonilla, L. Melatonin protects mice infected with Venezuelan equine encephalomyelitis virus. Cell. Mol. Life Sci. CMLS 1997, 53, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Nejati Moharrami, N.; Bjørkøy Tande, E.; Ryan, L.; Espevik, T.; Boyartchuk, V. RORα controls inflammatory state of human macrophages. PLoS ONE 2018, 13, e0207374. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Monté, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.-C.; Staels, B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef]
- Gold, M.J.; Antignano, F.; Halim, T.Y.F.; Hirota, J.A.; Blanchet, M.-R.; Zaph, C.; Takei, F.; McNagny, K.M. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J. Allergy Clin. Immunol. 2014, 133, 1142–1148.e1145. [Google Scholar] [CrossRef]
- Halim Timotheus, Y.F.; MacLaren, A.; Romanish Mark, T.; Gold Matthew, J.; McNagny Kelly, M.; Takei, F. Retinoic-Acid-Receptor-Related Orphan Nuclear Receptor Alpha Is Required for Natural Helper Cell Development and Allergic Inflammation. Immunity 2012, 37, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Lo, B.C.; Gold, M.J.; Hughes, M.R.; Antignano, F.; Valdez, Y.; Zaph, C.; Harder, K.W.; McNagny, K.M. The orphan nuclear receptor ROR alpha and group 3 innate lymphoid cells drive fibrosis in a mouse model of Crohn’s disease. Sci. Immunol. 2016, 1, eaaf8864. [Google Scholar] [CrossRef] [Green Version]
- Friesenhagen, J.; Viemann, D.; Börgeling, Y.; Schmolke, M.; Spiekermann, C.; Kirschnek, S.; Ludwig, S.; Roth, J. Highly Pathogenic Influenza Viruses Inhibit Inflammatory Response in Monocytes via Activation of Rar-Related Orphan Receptor RORa. J. Innate Immun. 2013, 5, 505–518. [Google Scholar] [CrossRef]
- Yanuck, S.F.P.; Messier, H.J.; Fitzgerald, K.N. Evidence Supporting a Phased Immuno-physiological Approach to COVID-19 from Prevention through Recovery. Integr. Med. 2020, 19, 8–35. [Google Scholar]
- Narimanian, M.; Badalyan, M.; Panosyan, V.; Gabrielyan, E.; Panossian, A.; Wikman, G.; Wagner, H. Impact of Chisan® (ADAPT-232) on the quality-of-life and its efficacy as an adjuvant in the treatment of acute non-specific pneumonia. Phytomedicine 2005, 12, 723–729. [Google Scholar] [CrossRef] [PubMed]


| Virus | AP Andrographolides | ES Eleutherosides | GS Glycyrrhizin and Glycyrrhizic Acid | PS Ginsenosides | RR Salidroside, Rosavin, Ellagic and Gallic Acids | SC Schisandrins and Anwulignan | WS Withanolides |
|---|---|---|---|---|---|---|---|
| SARS-related coronavirus | [31,32,33] | ||||||
| Ebola virus (EBOV) and Marburg virus (MARV) | [34] | ||||||
| Human rhinovirus (HRV) | [35] | ||||||
| Respiratory syncytial virus (RSV) | [35] | [36] | [37] | ||||
| H1N1 influenza A virus | [38,39,40] | [35,41,42,43,44] | [40,45] | [46,47,48,49,50,51,52,53] | [54] | ||
| H2N2 influenza virus | [55] | ||||||
| H3N2 influenza virus | [50,53,56] | ||||||
| H5N1 avian influenza virus | [57] | [58] | [50,56] | [34] | |||
| H7N9 influenza | [50] | ||||||
| H9N2 avian influenza virus | [54] | ||||||
| Chikungunya virus | [59,60,61] | ||||||
| Dengue virus | [59,60] | [62] | |||||
| Coxsackievirus B3 | [63] | [64] |
| Target/Mediator | AP Andrographolides | ES Eleutherosides | GS Glycyrrhizin and Glycyrrhizic Acid | PS Ginsenosides | RR Salidroside, Rosavin, Ellagic and Gallic Acids | SC Schisandrins and Anwulignan | WS Withanolides |
|---|---|---|---|---|---|---|---|
| Effects on Viral Life Cycle in Infected Host Cells—Targets Preventing the Virus RNA Synthesis and Replication | |||||||
| Nsp5: 3-chymotrypsin-like protease (3Clpro)–main protease of SARS-CoV-2 (Mpro) | [22,65] | [19,66] | [19,66] | [19,66] | [19,66] | ||
| Nsp3: Papain-like protease (Plpro) | [22] | [19,66] | [19,66] | [19,66] | [19,66] | ||
| Nsp12: RNA-dependent RNA polymerase (RdRp) | [22] | ||||||
| Nsp1: The most N-terminal gene 1 protein | [22] | ||||||
| Targets Inhibiting Virus Structural Proteins | |||||||
| S1: Spike glycoprotein binding SARS-CoV-2 to human angiotensin-converting enzyme 2 (ACE2) of host cells | [67] | ||||||
| S2: Spike glycoprotein receptor to type-II transmembrane serine protease enzymes (TMPRSS2) of host cells | [22] | [67] | |||||
| Blockage of binding viral (Ebola/Marburg) surface glycoproteins to host cells | [34] | ||||||
| Target/Mediator | AP Andrographolides | ES Eleutherosides | GS Glycyrrhizin and Glycyrrhizic Acid | PS Ginsenosides | RR Salidroside, Rosavin, Ellagic and Gallic Acids | SC Schisandrins and Anwulignan | WS Withanolides | Melatonin |
|---|---|---|---|---|---|---|---|---|
| Innate Immunity | ||||||||
| Defensins: Human β-defensin-2 | [68,69] | |||||||
| Pathogen’s pattern recognition via TLR | [70,71] | [11,72] | [67,73,74,75,76,77] | [78,79,80,81,82,83] | [11,84,85] | [11,86,87,88] | [11,89] | [90,91,92,93,94] |
| Interferons | [95] | [96,97,98,99,100,101,102] | [33,55] | [37,46,48,49,56] | [62,63] | [86] | [103,104,105,106] | [90] |
| Natural killer cells | [97,98] | [58] | [46] | |||||
| Interleukins: IL-6, IL-1β, IL-10, TNF, etc. | [95,107,108] | [108,109] | [74,77,110] | [37,48,49,52,111,112] | [62,63,84] | [86,110,113] | [103,104,105,106,114] | [90,92,94,115] |
| Melatonin signaling pathways | [11] | [11] | [11] | [11] | [11] | |||
| Adaptive Immunity | ||||||||
| T cells and MHC proteins | [97,98,99,116] | [33,55,58] | [86] | [103,104,105,106,117] | ||||
| B cells and antibodies | [95] | [72] | [55] | [104,105,106] | ||||
| Target/Mediator | AP Andrographolides | ES Eleutherosides | GS Glycyrrhizin and Glycyrrhizic Acid | PS Ginsenosides | RR Salidroside, Rosavin, Ellagic and Gallic Acids | SC Schisandrins and Anwulignan | WS Withanolides | Melatonin |
|---|---|---|---|---|---|---|---|---|
| Arachidonic acid release, inhibition of phospholipase 2 | [118] | [119,120,121] | [122,123,124,125] | [126] | [127] | [128,129,130] | ||
| COX-2-mediated signaling | [71,131] | [132] | [74,119,121] | [111,123,125] | [132] | [132] | [132] | |
| Lipoxygenase-mediated signaling of arachidonic acid pro- and anti-inflammatory metabolites leukotrienes, lipoxins, resolvins, etc. | [132] | [119,121] | [132] | [127] | [130,132] | [132] | ||
| Platelet-activating factor (PAF) | [133,134] | [135,136] | [137,138] | [89] | ||||
| Nitric oxide-mediated inflammation: Inducible NO synthase oxide catabolites (NOCs) | [108,139] | [108] | [32,74,108] | [52] | [140] | [88,108,140] | [90,115] | |
| NF-κB-mediated inflammation NF-κB signaling, translocation and expression | [70,71,107,141] | [72,142,143,144,145,146,147,148,149,150] | [74,77] | [151,152,153,154,155] | [156,157,158,159,160,161,162,163] | [86,87,113,164,165,166,167,168] | [89,114,169,170,171,172] | [90,173] |
| Nrf2-mediated oxidative stress response signaling pathway proteins: Phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), KEAP1, etc. Nrf2-ARE (antioxidant response element) expression | [174,175,176,177,178,179,180,181,182] | [183] | [119] | [111,151,184,185,186,187] | [159,160,162,163,188,189,190] | [86,113,165,191,192,193] | [194,195,196] | [197] |
| Antioxidant proteins (SOD, GST, NQO1 and HO1), lipid peroxidation | [176,178,182] | [183] | [111,151,185,187,198] | [158,160,162,163,188,190] | [86,113,165,191,192,193] | [103,194] | [93,115,197] | |
| Molecular chaperon-mediated cytoprotectant and repair processes Heat shock proteins Hsp72 | [199,200] | [140,199,200,201] | [140,199,200,201] | |||||
| Melatonin signaling Retinoic acid receptor (RAR)-related orphan nuclear receptor alpha (RORα) | [11] | [11] | [11] | [11] | [11,202,203] |
| Activity | AP | ES | GS | PS | RR | SC | WS |
|---|---|---|---|---|---|---|---|
| adaptogenic | [204,205] | [206] | [207] | [208] | [209] | [206,210,211,212,213] | [214,215,216] |
| antidiabetic | [217,218,219,220] | [221] | [212] | [214,215,216] | |||
| antioxidant | [222,223,224] | [221] | [207] | [208] | [209] | [210,211,213] | [214,215,216] |
| immunomodulatory | [225] | [221] | [207] | [208] | [210,211,213] | [214,215,216] | |
| metabolism | [207] | [208] | [209] | [212] | |||
| gastroprotective | [226,227] | [207] | [212] | ||||
| hepatoprotective | [228,229,230,231] | [221] | [207] | [208] | [210,211,213] | [214,215,216] | |
| cardioprotective | [232,233] | [221] | [208] | [209] | [210,211,213] | [214,215,216] | |
| antiproliferative | [234,235,236] | [221] | [207] | [208] | [209] | [210,211,213] | |
| neuroprotective | [237] | [221] | [208] | [209] | [210,211,213] | [214,215,216] | |
| anti-stress/anti-fatigue | [238] | [221] | [208] | [209] | [210,211,213] | [214,215,216] | |
| antidepressant | [239] | [221] | [207] | [208] | [240,241] | [212] | [214,215,216] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13090236
Panossian A, Brendler T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals. 2020; 13(9):236. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13090236
Chicago/Turabian StylePanossian, Alexander, and Thomas Brendler. 2020. "The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections" Pharmaceuticals 13, no. 9: 236. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13090236