Lenvatinib for Hepatocellular Carcinoma: A Literature Review
Abstract
:1. Introduction
2. Therapeutic Response of Lenvatinib
2.1. The Ojective Response Rate of Lenvatinib
2.2. The Progression-Free Survival and Overall Survival
3. Adverse Events
3.1. The Fatigue, Appetite Loss and Treatment Discontinuation
3.2. The Hemorrhage Events
3.3. Hepatic Encephalopathy and Ascites
3.4. Thyroid Dysfunction
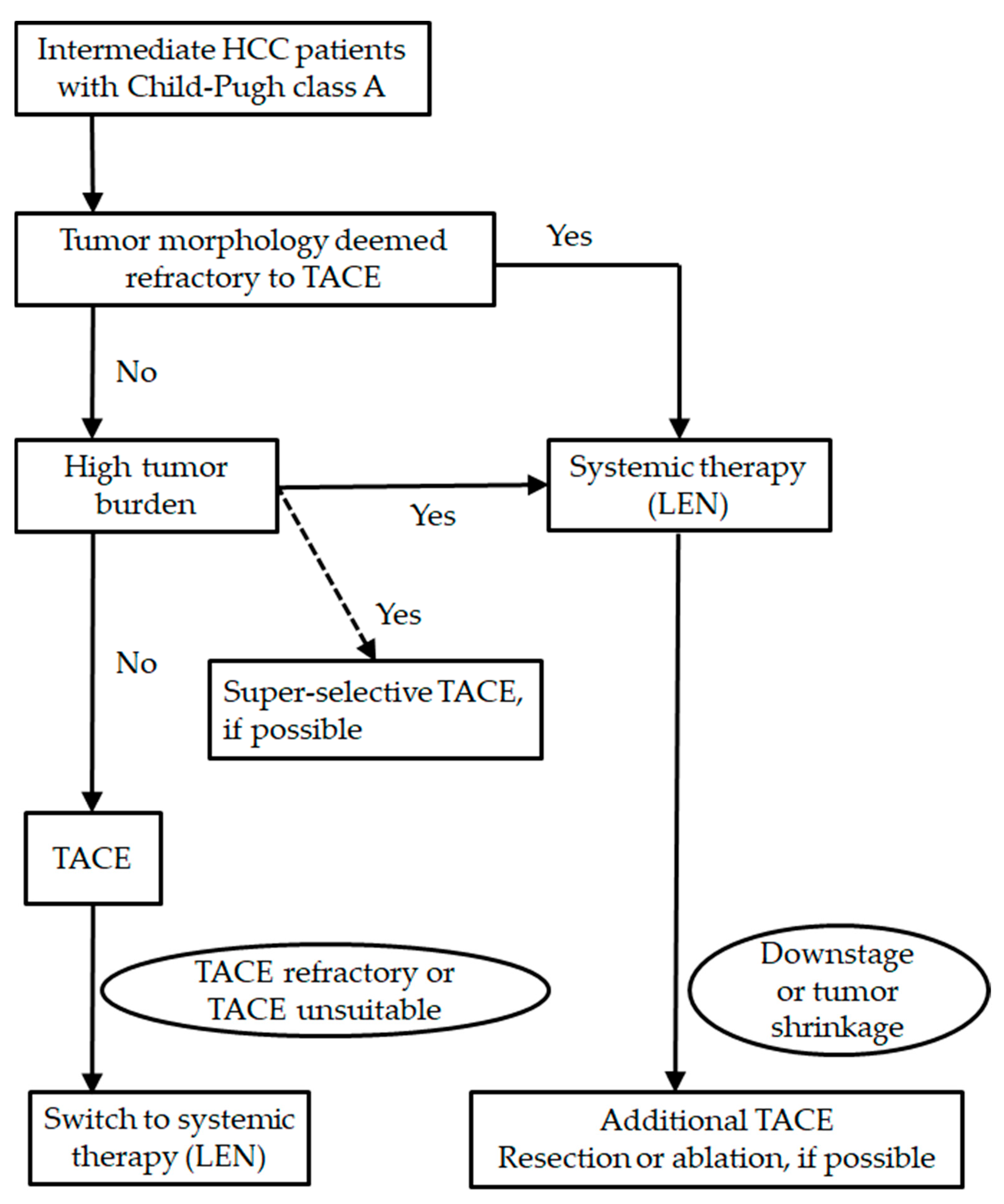
4. Lenvatinib for Patients with Barcelona Clinic Liver Cancer Intermediate Stage
4.1. Transcatheter Arterial Chemoembolization and Heterogeneity
4.2. Transcatheter Arterial Chemoembolization Refractoriness
4.3. Exceeding the up-to Seven Criteria, Transcatheter Arterial Chemoembolization Unsuitability and Upfront Systemic Therapies
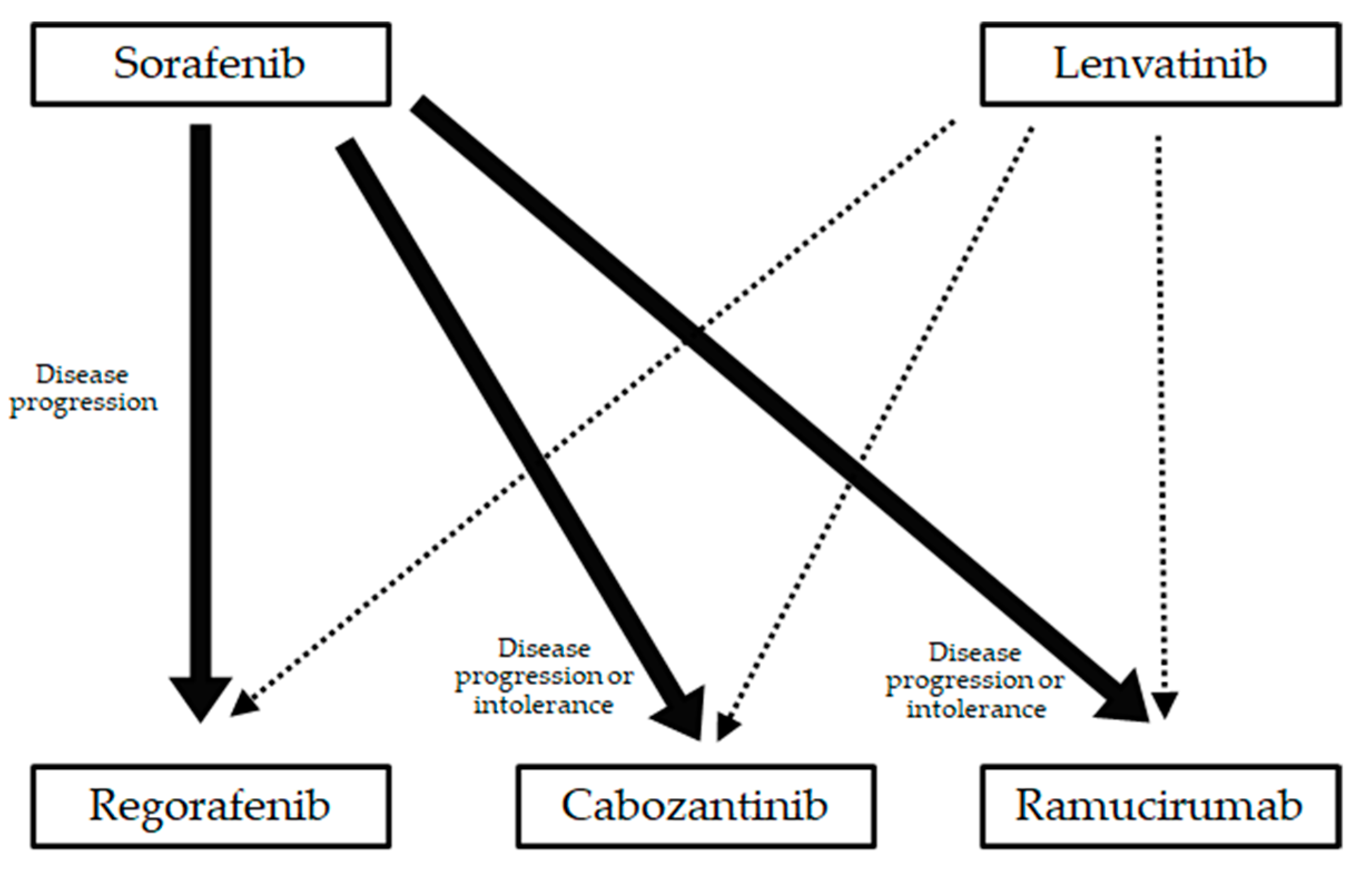
5. Post-Progression Treatment after Lenvatinib
5.1. Changes in the Liver Function during Lenvatinib Treatment
5.2. The Frequency and Predictive Factors of Transition to Post-Progression Treatment
5.3. The Comparison of Sorafenib and Lenvatinib Treatment
6. Nutrition Assessment and Sarcopenia
6.1. Nutrition Assessment as a Prognostic Indicator
6.2. The Relationship between Sarcopenia and Overall Survival
7. Lenvatinib plus Pembrolizumab Therapy
8. Efficacy and Safety in Patients Excluded from the REFLECT Study
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014, 87 (Suppl. 1), 22–31. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef] [Green Version]
- Tohyama, O.; Matsui, J.; Kodama, K.; Hata-Sugi, N.; Kimura, T.; Okamoto, K.; Minoshima, Y.; Iwata, M.; Funahashi, Y. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid. Res. 2014, 2014, 638747. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 2014, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: A multicenter study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, T.; Kakizaki, S.; Nagashima, T.; Namikawa, M.; Tojima, H.; Shimada, Y.; Takizawa, D.; Naganuma, A.; Arai, H.; Sato, K.; et al. Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: A multicenter retrospective study. Hepatol. Res. 2020, 50, 382–395. [Google Scholar] [CrossRef]
- Sasaki, R.; Fukushima, M.; Haraguchi, M.; Miuma, S.; Miyaaki, H.; Hidaka, M.; Eguchi, S.; Matsuo, S.; Tajima, K.; Matsuzaki, T.; et al. Response to lenvatinib is associated with optimal relativedose intensity in hepatocellular carcinoma: Experience in clinical settings. Cancers 2019, 11, 1769. [Google Scholar] [CrossRef] [Green Version]
- Sho, T.; Suda, G.; Ogawa, K.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; Umemura, M.; Kawagishi, N.; Natsuizaka, M.; et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol. Res. 2020, 50, 966–977. [Google Scholar] [CrossRef]
- Ogushi, K.; Chuma, M.; Uojima, H.; Hidaka, H.; Numata, K.; Kobayashi, S.; Hirose, S.; Hattori, N.; Fujikawa, T.; Nakazawa, T.; et al. Safety and efficacy of lenvatinib treatment in Child-Pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: A multicenter analysis. Clin. Exp. Gastroenterol. 2020, 13, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Maruta, S.; Ogasawara, S.; Ooka, Y.; Obu, M.; Inoue, M.; Itokawa, N.; Haga, Y.; Seki, A.; Okabe, S.; Azemoto, R.; et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer 2020, 9, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Sato, K.; Kondo, M.; Goto, E.; Sato, T.; Kondo, Y.; Akamatsu, M.; Sato, S.; Yoshida, H.; Koike, Y.; et al. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: A multicenter retrospective study. Drugs Real World Outcomes 2020, 7, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 2019, 8, 3719–3728. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.X.; Yang, X.; Lin, J.Z.; Bai, Y.; Long, J.Y.; Yang, X.B.; Seery, S.; Zhao, H.T. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: A retrospective, real-world study conducted in China. World J. Gastroenterol. 2020, 26, 4465–4478. [Google Scholar] [CrossRef]
- Cheon, J.; Chon, H.J.; Bang, Y.; Park, N.H.; Shin, J.W.; Kim, K.M.; Lee, H.C.; Lee, J.; Yoo, C.; Ryoo, B.Y. Real-World efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: A multicenter retrospective analysis. Liver Cancer 2020, 9, 613–624. [Google Scholar] [CrossRef]
- Kudo, M. Extremely high objective response rate of lenvatinib: Its clinical relevance and changing the treatment paradigm in hepatocellular carcinoma. Liver Cancer 2018, 7, 215–224. [Google Scholar] [CrossRef]
- Kirino, S.; Tsuchiya, K.; Kurosaki, M.; Kaneko, S.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Okada, M.; et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS ONE 2020, 15, e0231828. [Google Scholar] [CrossRef] [Green Version]
- Ono, A.; Aikata, H.; Yamauchi, M.; Kodama, K.; Ohishi, W.; Kishi, T.; Ohya, K.; Teraoka, Y.; Osawa, M.; Fujino, H.; et al. Circulating cytokines and angiogenic factors based signature associated with the relative dose intensity during treatment in patients with advanced hepatocellular carcinoma receiving lenvatinib. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922051. [Google Scholar] [CrossRef]
- Takahashi, A.; Moriguchi, M.; Seko, Y.; Ishikawa, H.; Yo, T.; Kimura, H.; Fujii, H.; Shima, T.; Mitsumoto, Y.; Ishiba, H.; et al. Impact of relative dose intensity of early-phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 2019, 39, 5149–5156. [Google Scholar] [CrossRef]
- Eso, Y.; Nakano, S.; Mishima, M.; Arasawa, S.; Iguchi, E.; Nakamura, F.; Takeda, H.; Takai, A.; Takahashi, K.; Taura, K.; et al. Dose intensity/body surface area ratio is a novel marker useful for predicting response to lenvatinib against hepatocellular carcinoma. Cancers 2019, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Kuorda, H.; Abe, T.; Fujiwara, Y.; Okamoto, T.; Yonezawa, M.; Sato, H.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Kawaoka, T.; Namba, M.; Uchikawa, S.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Yamauchi, M.; Hiramatsu, A.; et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 2019, 97, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol. Res. 2020, 50, 374–381. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kakizaki, S.; Nagashima, T.; Namikawa, M.; Ueno, T.; Tojima, H.; Takizawa, D.; Naganuma, A.; Arai, H.; Sato, K.; et al. Liver function changes in patients with hepatocellular carcinoma treated with lenvatinib: Predictive factors of progression to Child-pugh class B, the formation of ascites and the candidates for the post-progression treatment. Cancers 2020, 12, 2906. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. C-reactive protein can predict dose intensity, time to treatment failure and overall survival in HCC treated with lenvatinib. PLoS ONE 2020, 15, e0244370. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Yu, S.; Mamtani, R.; Mehta, R.; D’Addeo, K.; Wileyto, E.P.; Taddei, T.H.; Kaplan, D.E. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: A retrospective, multi-institutional study. J. Clin. Oncol. 2017, 35, 3575–3581. [Google Scholar] [CrossRef]
- Yamashita, T.; Kudo, M.; Ikeda, K.; Izumi, N.; Tateishi, R.; Ikeda, M.; Aikata, H.; Kawaguchi, Y.; Wada, Y.; Numata, K.; et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: An analysis of Japanese subset. J. Gastroenterol. 2020, 55, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallanzani, A.; Orsi, G.; Andrikou, K.; Gelsomino, F.; Rimini, M.; Riggi, L.; Cascinu, S. Lenvatinib as a therapy for unresectable hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 1069–1076. [Google Scholar] [CrossRef]
- Shimose, S.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; Suga, H.; Kuromatsu, R.; et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: A multicenter retrospective study. Cancers 2020, 12, 1867. [Google Scholar] [CrossRef]
- Iwamoto, H.; Suzuki, H.; Shimose, S.; Niizeki, T.; Nakano, M.; Shirono, T.; Okamura, S.; Noda, Y.; Kamachi, N.; Nakamura, T.; et al. Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers 2020, 12, 1010. [Google Scholar] [CrossRef]
- Okubo, H.; Ando, H.; Ishizuka, K.; Kitagawa, R.; Okubo, S.; Saito, H.; Kokubu, S.; Miyazaki, A.; Ikejima, K.; Shiina, S.; et al. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0229772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol. Res. 2020, 50, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Je, Y.; Schutz, F.A.; Choueiri, T.K. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009, 10, 967–974. [Google Scholar] [CrossRef]
- Schutz, F.A.; Je, Y.; Richards, C.J.; Choueiri, T.K. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J. Clin. Oncol. 2012, 30, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, J.; Wu, Z.; Tu, J.; Hu, Y.; Wu, S.; Lou, L. Fatal adverse events with molecular targeted agents in the treatment of advanced hepatocellular carcinoma: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2018, 12, 3043–3049. [Google Scholar] [CrossRef] [Green Version]
- Sahu, S.K.; Chawla, Y.K.; Dhiman, R.K.; Singh, V.; Duseja, A.; Taneja, S.; Kalra, N.; Gorsi, U. Rupture of hepatocellular carcinoma: A review of literature. J. Clin. Exp. Hepatol. 2019, 9, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M.; Izumi, N.; Kubo, S.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2020, 50, 15–46. [Google Scholar] [CrossRef] [Green Version]
- Uchida-Kobayashi, S.; Kageyama, K.; Yamamoto, A.; Ikenaga, H.; Yoshida, K.; Kotani, K.; Kimura, K.; Odagiri, N.; Hagihara, A.; Fujii, H.; et al. Lenvatinib-induced tumor-related hemorrhages in patients with large hepatocellular carcinomas. Oncology 2020, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kokudo, N.; Matsuyama, Y.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: An analysis of 1160 cases from a nationwide survey. Ann. Surg. 2014, 259, 532–542. [Google Scholar] [CrossRef]
- Ohya, K.; Kawaoka, T.; Namba, M.; Uchikawa, S.; Kodama, K.; Morio, K.; Nakahara, T.; Murakami, E.; Hiramatsu, A.; Tsuge, M.; et al. Early changes in ammonia levels and liver function in patients with advanced hepatocellular carcinoma treated by lenvatinib therapy. Sci. Rep. 2019, 9, 12101. [Google Scholar] [CrossRef] [PubMed]
- Narita, R.; Kotoh, K.; Yoneda, A.; Motomura, M.; Harada, M. Factors raising serum ammonia level during lenvatinib treatment of patients with hepatocellular carcinoma. Anticancer Res. 2020, 40, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Uojima, H.; Nakazawa, T.; Shao, X.; Hara, Y.; Iwasaki, S.; Wada, N.; Kubota, K.; Tanaka, Y.; Shibuya, A.; et al. Portal hemodynamic effects of lenvatinib in patients with advanced hepatocellular carcinoma: A prospective cohort study. Hepatol. Res. 2020, 50, 1083–1090. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Torino, F.; Corsello, S.M.; Longo, R.; Barnabei, A.; Gasparini, G. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009, 6, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, Y.; Hirooka, M.; Hiraoka, A.; Ochi, H.; Tanaka, T.; Yukimoto, A.; Imai, Y.; Watanabe, T.; Yoshida, O.; Miyake, T.; et al. Lenvatinib-induced thyroid abnormalities in unresectable hepatocellular carcinoma. Endocr. J. 2019, 66, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Shomura, M.; Okabe, H.; Sato, E.; Fukai, K.; Shiraishi, K.; Hirose, S.; Tsuruya, K.; Arase, Y.; Anzai, K.; Kagawa, T. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers 2020, 12, 3078. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montana, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Sola, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, S.; Kelly, D.; O’Kane, G.M. Non-immunotherapy options for the first-line management of hepatocellular carcinoma: Exploring the evolving role of sorafenib and lenvatinib in advanced disease. Curr. Oncol. 2020, 27, S165–S172. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [PubMed]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: Proposal of modified Bolondi’s subclassification (Kinki Criteria). Dig. Dis. 2015, 33, 751–758. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.J.; Park, M.S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J. Hepatol. 2015, 62, 1304–1310. [Google Scholar] [CrossRef]
- Golfieri, R.; Renzulli, M.; Mosconi, C.; Forlani, L.; Giampalma, E.; Piscaglia, F.; Trevisani, F.; Bolondi, L. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: An issue of nodule dimension? J. Vasc. Interv. Radiol. 2013, 24, 509–517. [Google Scholar] [CrossRef]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Kanai, F.; Yoshikawa, M.; Yokosuka, O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014, 87, 330–341. [Google Scholar] [CrossRef]
- Arizumi, T.; Ueshima, K.; Minami, T.; Kono, M.; Chishina, H.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer 2015, 4, 253–262. [Google Scholar] [CrossRef]
- Kudo, M.; Raoul, J.-L.; Lee, H.C.; Cheng, A.-L.; Nakajima, K.; Peck-Radosavljevic, M. Deterioration of liver function after transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): An exploratory analysis of OPTIMIS—An international observational study assessing the use of sorafenib after TACE. J. Clin. Oncol. 2018, 36, 368. [Google Scholar] [CrossRef]
- Shimose, S.; Kawaguchi, T.; Tanaka, M.; Iwamoto, H.; Miyazaki, K.; Moriyama, E.; Suzuki, H.; Niizeki, T.; Shirono, T.; Nakano, M.; et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol. Lett. 2020, 20, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Eso, Y.; Takai, A.; Takahashi, K.; Ueda, Y.; Taura, K.; Marusawa, H.; Seno, H. Combination of Mac-2 binding protein glycosylation isomer and up-to-seven criteria as a useful predictor for Child-Pugh grade deterioration after transarterial chemoembolization for hepatocellular carcinoma. Cancers 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasui, Y.; Tsuchiya, K.; Kurosaki, M.; Takeguchi, T.; Takeguchi, Y.; Okada, M.; Wang, W.; Kubota, Y.; Goto, T.; Komiyama, Y.; et al. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol. Res. 2018, 48, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh A liver function: A proof-of-concept study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial design and endpoints in hepatocellular carcinoma: AASLD Consensus Conference. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Early relative change in hepatic function with lenvatinib for unresectable hepatocellular carcinoma. Oncology 2019, 97, 334–340. [Google Scholar] [CrossRef]
- Personeni, N.; Pressiani, T.; Rimassa, L. Lenvatinib for the treatment of unresectable hepatocellular carcinoma: Evidence to date. J. Hepatocell. Carcinoma 2019, 6, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Terashima, T.; Yamashita, T.; Takata, N.; Nakagawa, H.; Toyama, T.; Arai, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; et al. Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol. Res. 2016, 46, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsina, A.; Kudo, M.; Vogel, A.; Cheng, A.L.; Tak, W.Y.; Ryoo, B.Y.; Evans, T.R.J.; López López, C.; Daniele, B.; Misir, S.; et al. Effects of subsequent systemic anticancer medication following first-line lenvatinib: A post hoc responder analysis from the phase 3 REFLECT study in unresectable hepatocellular carcinoma. Liver Cancer 2020, 9, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; et al. Post-progression treatment eligibility of unresectable hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer 2020, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Kawaoka, T.; Suehiro, Y.; Yamaoka, K.; Kosaka, Y.; Uchikawa, S.; Kodama, K.; Morio, K.; Fujino, H.; Nakahara, T.; et al. Analysis of post-progression survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Oncology 2020, 98, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 2019, 97, 277–285. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Initial experience of ramucirumab treatment after lenvatinib failure for patients with advanced hepatocellular carcinoma. Anticancer Res. 2020, 40, 2089–2093. [Google Scholar] [CrossRef]
- Kasuya, K.; Kawamura, Y.; Kobayashi, M.; Shindoh, J.; Kobayashi, Y.; Kajiwara, A.; Iritani, S.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; et al. Efficacy and safety of ramucirumab in patients with unresectable hepatocellular carcinoma with progression after treatment with lenvatinib. Intern. Med. 2020, 5185-20. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Takata, N.; Toyama, T.; Shimakami, T.; Takatori, H.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; et al. Comparative analysis of liver functional reserve during lenvatinib and sorafenib for advanced hepatocellular carcinoma. Hepatol. Res. 2020, 50, 871–884. [Google Scholar] [CrossRef]
- Nakano, M.; Kuromatsu, R.; Niizeki, T.; Okamura, S.; Iwamoto, H.; Shimose, S.; Shirono, T.; Noda, Y.; Kamachi, N.; Koga, H.; et al. Primary Treatment with Molecular-Targeted Agents for Hepatocellular Carcinoma: A Propensity Score-matching Analysis. Hepatol. Commun. 2020, 4, 1218–1228. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Cheng, A.L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020, 40, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Platelet-lymphocyte ratio predicts survival in patients with hepatocellular carcinoma who receive lenvatinib: An inverse probability weighting analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Shimose, S.; Kawaguchi, T.; Iwamoto, H.; Tanaka, M.; Miyazaki, K.; Ono, M.; Niizeki, T.; Shirono, T.; Okamura, S.; Nakano, M.; et al. Controlling nutritional status (CONUT) score is associated with overall survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib: A multicenter cohort study. Nutrients 2020, 12, 1076. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Chan, A.W.; Chan, S.L.; Wong, G.L.; Wong, V.W.; Chong, C.C.; Lai, P.B.; Chan, H.L.; To, K.F. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann. Surg. Oncol. 2015, 22, 4138–4148. [Google Scholar] [CrossRef]
- Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. The optimal cut-off value of the preoperative prognostic nutritional index for the survival differs according to the TNM stage in hepatocellular carcinoma. Surg. Today 2017, 47, 986–993. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kakizaki, S.; Uehara, D.; Nagashima, T.; Ueno, T.; Namikawa, M.; Saito, S.; Hosonuma, K.; Suzuki, H.; Naganuma, A.; et al. Impact of the prognostic nutritional index on the survival of Japanese patients with hepatocellular carcinoma treated with sorafenib: A multicenter retrospective study. Intern. Med. 2019, 58, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Caputo, F.; Dadduzio, V.; Tovoli, F.; Bertolini, G.; Cabibbo, G.; Cerma, K.; Vivaldi, C.; Faloppi, L.; Rizzato, M.D.; Piscaglia, F.; et al. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with Sorafenib. PLoS ONE 2020, 15, e0232449. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Nutritional index as prognostic indicator in patients receiving lenvatinib treatment for unresectable hepatocellular carcinoma. Oncology 2020, 98, 295–302. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Uojima, H.; Chuma, M.; Tanaka, Y.; Hidaka, H.; Nakazawa, T.; Iwabuchi, S.; Kobayashi, S.; Hattori, N.; Ogushi, K.; Morimoto, M.; et al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer 2020, 9, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Kuroda, H.; Kanazawa, J.; Sato, T.; Fujiwara, Y.; Abe, T.; Sato, H.; Kooka, Y.; Oikawa, T.; Sawara, K.; et al. Impact of grip strength in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Cancers 2020, 12, 2146. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Takaguchi, K.; Itobayashi, E.; Shimada, N.; Tajiri, K.; Tsuji, K.; Ishikawa, T.; Ochi, H.; et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis. Hepatol. Res. 2019, 49, 111–117. [Google Scholar] [CrossRef] [Green Version]
| Author/Ref. No. | Years | Country | No. of pts | ORR (%) | Median PFS (Months) | Median OS (Months) | Predictive Factors of ORR | Commentary | |
|---|---|---|---|---|---|---|---|---|---|
| mRECIST | RECIST ver.1.1 | ||||||||
| Kudo M./[12] | 2018 | Global | 478 | 40.6 | 18.8 | 7.3 | 13.6 | NA | REFLECT trial |
| Ueshima K./[17] | 2019 | Japan | 82 | 39.0 | NA | 7.6 | could not be reached | ALBI grade 1 | ALBI grade 1; ORR of 57.1%, median PFS of 18.9 months |
| Hatanaka T./[18] | 2020 | Japan | 94 | 30.4 | NA | 5.4 | NA | BCLC intermediate stage | BCLC intermediate stage; ORR of 47.6%, median PFS 8.0 months |
| Sasaki R./[19] | 2019 | Japan | 81 | 34.6 | 17.3 | NA | 11.6 | NA | The patients with the good ORR and those with high RDI had significantly good OS. |
| Sho T./[20] | 2020 | Japan | 105 | 53.5 | NA | 9.8 | NA | NA | |
| Ogushi K./[21] | 2020 | Japan | 181 | 30.4 | NA | NA | 369 (days) | CP-A, PS 0, RDI > 0.70 | CP-A; ORR of 36.5%. RDI > 0.70: ORR of 47.7%. CP-A and BCLC intermediate stage were the predictive factors affecting the OS. |
| Maruta S./[22] | 2020 | Japan | 152 | 40.8 | 15.8 | 5.1 | 13.3 | NA | |
| Ohki T./[23] | 2020 | Japan | 77 | 29.9 | NA | 5.6 | NA | CP-A, RDI > 70% at 30 days, AFP reduction | RDI > 70%; ORR of 45.2%, RDI ≤ 70%; ORR of 11.4%. The significant factors associated with the PFS were CP-5A, tumor size ≥ 40 mm among pretreatment factors. |
| Hiraoka A./[24] | 2019 | Japan | 152 | 38.7 | NA | NA | could not be reached | NA | mALBI 2b or 3 was unfavorable predictive factor relevant to the OS. |
| Wang D.X./[25] | 2020 | China | 54 | NA | 22.2 | 5.6 | could not be reached | could not be found in a multivariate analysis. | CP-A, BCLC intermediate stage and PVTT significantly affected the PFS. |
| Cheon J./[26] | 2020 | Korea | 92 | NA | 14.1 | 4.3 | 7.1 | NA | |
| Author/Ref. No. | Years | No. of pts | Assessment | Time | Cut-off Value | Associated Factors | Therapeutic Response | Commentary |
|---|---|---|---|---|---|---|---|---|
| Sasaki R./[19] | 2019 | 81 | RDI | 8 weeks | 67% | BMI, PS, BCLC stage, platelet count, PT, albumin, initial dose | OS | Median OS could not be reached during the observation. |
| Ogushi K./[21] | 2020 | 181 | RDI | 8 weeks | 70% | NA | ORR | Mean RDI gradually decreased from the start of lenvatinib to 2 months. |
| Ohki T./[23] | 2020 | 77 | RDI | 30 days | 70% | NA | ORR, PFS | RDI > 70%; median PFS of 9.3 months |
| Kirino S./[28] | 2020 | 60 | RDI | 4 weeks | 70% | Body weight, ALBI score, albumin, grade 3 or 4 adverse events | OS, time to discontinuation of treatment | Median OS could not be reached and median duration of lenvatinib was 9.5 months in patients with RDI > 70%. |
| Ono A./[29] | 2020 | 41 | RDI | 4 weeks | 70% | Platelet count, albumin, AST, AFP-L3, DCP, ALBI score, TKI experience | PFS | RDI > 70%; median PFS of 6.7 months. RDI in 3–4 weeks significantly reduced compared to 1–2 weeks. |
| Takahashi A./[30] | 2019 | 50 | RDI | 8 weeks | 75% | Child-Pugh class A, ALBI, EHS, initial dose | ORR, PFS | RDI > 75%; ORR of 68%, median PFS of 7.4 months |
| Eso Y./[31] | 2019 | 49 | DBR, RDI | 60 days | DBR of 238.9, RDI of 66% | BSA, mALBI grade 1 + 2a, BTR | ORR, PFS | DBR was calculated as the delivered DI divided by BSA. |
| Author/Ref. No. | Years | No. of pts | Frequency of Post-Progression Treatment | Frequency of Ramucirumab Treatment | Favorable Predictive Factors of Post-Progression Treatment |
|---|---|---|---|---|---|
| Hatanaka T./[35] | 2020 | 79 | 45.6% | 13.9% | ALBI grade 1, Child-Pugh score 5 |
| Hiraoka A./[83] | 2020 | 73 | 43.8% | 20.0% | mALBI grade 1 + 2a |
| Ando Y./[84] | 2020 | 53 | 50.9% | NA | mALBI grade 1 + 2a |
| Author/Ref. No. | Years | No. of pts | Assessment | Cut-off Value |
|---|---|---|---|---|
| Tada T./[92] | 2020 | 237 | NLR | 4 |
| Tada T./[93] | 2020 | 283 | PLR | 150 |
| Shimose S./[95] | 2020 | 164 | CONUT score | 5 |
| Hiraoka A./[101] | 2020 | 375 | PNI | 40 |
| Uojima H./[103] | 2020 | 100 | SMI | 42 and 38 cm2/m2 in men and women, respectively |
| Endo K./[104] | 2020 | 69 | grip strength | 26 kg and 18 kg in men and women, respectively |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatanaka, T.; Naganuma, A.; Kakizaki, S. Lenvatinib for Hepatocellular Carcinoma: A Literature Review. Pharmaceuticals 2021, 14, 36. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14010036
Hatanaka T, Naganuma A, Kakizaki S. Lenvatinib for Hepatocellular Carcinoma: A Literature Review. Pharmaceuticals. 2021; 14(1):36. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14010036
Chicago/Turabian StyleHatanaka, Takeshi, Atsushi Naganuma, and Satoru Kakizaki. 2021. "Lenvatinib for Hepatocellular Carcinoma: A Literature Review" Pharmaceuticals 14, no. 1: 36. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14010036






