Morphological Changes and Prognostic Factors before and after Photodynamic Therapy for Central Serous Chorioretinopathy
Abstract
:1. Introduction
2. Results
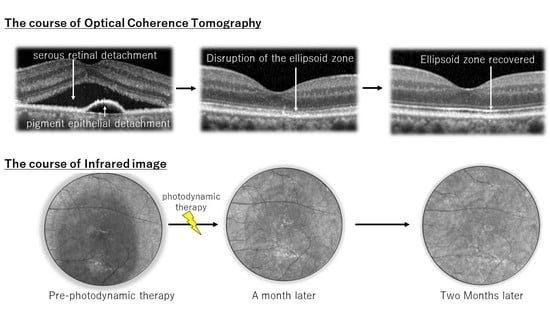
2.1. Changes in Luminance, Visual Acuity, Choroidal Morphology, and IR Images after as Compared to before hPDT
2.2. Prognostic Factors Associated with Treatment
3. Discussion
4. Materials and Methods
4.1. Statistical Analysis
4.2. Ethics Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gass, J.D. Pathogenesis of disciform detachment of the neuroepithelium. Am. J. Ophthalmol. 1967, 63, 573-1–585-13. [Google Scholar]
- Spaide, R.F.; Campeas, L.; Haas, A.; Yannuzzi, L.A.; Fisher, Y.L.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Orlock, D.A. Central serous chorioretinopathy in Younger and Older Adults. Ophthalmology 1992, 113, 522–528. [Google Scholar] [CrossRef]
- Kaye, R.; Chandra, S.; Sheth, J.; Boon, C.J.F.; Sivaprasad, S.; Lotery, A. Central serous chorioretinopathy: An update on risk factors, pathophysiology and imaging modalities. Prog. Retin. Eye Res. 2020, 79, 100865. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Brucker, A.J.; Robinson, F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology 1989, 96, 854–859. [Google Scholar] [CrossRef]
- Van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Mrejen, S.; Balaratnasingam, C.; Kaden, T.R.; Bottini, A.; Dansingani, K.; Bhavsar, K.V.; Yannuzzi, N.A.; Patel, S.; Chen, K.C.; Yu, S.; et al. Long-term Visual Outcomes and Causes of Vision Loss in Chronic Central Serous Chorioretinopathy. Ophthalmology 2019, 126, 576–588. [Google Scholar] [CrossRef]
- Breukink, M.B.; Dingemans, A.J.; den Hollander, A.I.; Keunen, J.E.; MacLaren, R.E.; Fauser, S.; Querques, G.; Hoyng, C.B.; Downes, S.M.; Boon, C.J. Chronic central serous chorioretinopathy: Long-term follow-up and vision-related quality of life. Clin. Ophthalmol. 2016, 11, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Sartini, F.; Menchini, M.; Posarelli, C.; Casini, G.; Figus, M. Bullous Central Serous Chorioretinopathy: A Rare and Atypical Form of Central Serous Chorioretinopathy. A Systematic Review. Pharmaceuticals 2020, 13, 221. [Google Scholar] [CrossRef]
- Sartini, F.; Figus, M.; Nardi, M.; Casini, G.; Posarelli, C. Non-resolving, recurrent and chronic central serous chorioretinopathy: Available treatment options. Eye 2019, 33, 1035–1043. [Google Scholar] [CrossRef]
- Cardillo Piccolino, F.; Eandi, C.M.; Ventre, L.; Rigault de la Longrais, R.C.; Grignolo, F.M. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003, 23, 752–763. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Slakter, J.S.; Gross, N.E.; Spaide, R.F.; Costa, D.; Huang, S.J.; Klancnik, J.M., Jr.; Aizman, A. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: A pilot study. Retina 2003, 23, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Noble, J.; Forooghian, F.; Meyerle, C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv. Ophthalmol. 2013, 58, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.I.; Glassman, A.R.; Aiello, L.P.; Chakravarthy, U.; Flaxel, C.J.; Spaide, R.F. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014, 121, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Chan, W.M.; Li, H.; Lai, R.Y.; Liu, D.T.; Lam, D.S. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: A short-term pilot study. Br. J. Ophthalmol. 2006, 90, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K.; Imamura, Y.; Shinoda, K.; Matsumoto, C.S.; Mizutani, Y.; Hashizume, K.; Mizota, A.; Yuzawa, M. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2015, 122, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Haga, F.; Maruko, R.; Sato, C.; Kataoka, K.; Ito, Y.; Terasaki, H. Long-term prognostic factors of chronic central serous chorioretinopathy after half-dose photodynamic therapy: A 3-year follow-up study. PLoS ONE 2017, 12, e0181479. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.M.; Lam, D.S.; Lai, T.Y.; Tam, B.S.; Liu, D.T.; Chan, C.K. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: A novel treatment at the primary disease level. Br. J. Ophthalmol. 2003, 87, 1453–1458. [Google Scholar] [CrossRef] [Green Version]
- Schlötzer-Schrehardt, U.; Viestenz, A.; Naumann, G.O.; Laqua, H.; Michels, S.; Schmidt-Erfurth, U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes. Arch. Clin. Exp. Ophthalmol. 2002, 240, 748–757. [Google Scholar] [CrossRef]
- Fujita, K.; Yuzawa, M.; Mori, R. Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy: Short-term results. Retina 2011, 31, 772–778. [Google Scholar] [CrossRef]
- Rabiolo, A.; Zucchiatti, I.; Marchese, A.; Baldin, G.; Sacconi, R.; Montorio, D.; Cicinelli, M.V.; Querques, L.; Bandello, F.; Querques, G. Multimodal retinal imaging in central serous chorioretinopathy treated with oral eplerenone or photodynamic therapy. Eye 2018, 32, 55–66. [Google Scholar] [CrossRef]
- Elsner, A.E.; Burns, S.A.; Weiter, J.J.; Delori, F.C. Infrared imaging of sub-retinal structures in the human ocular fundus. Vis. Res. 1996, 36, 191–205. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Chen, C.; Yi, Z.; Wang, X.; Liu, J.; Zheng, H. Clinical application of multicolor imaging in central serous chorioretinopathy. Retina 2020, 40, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Maruko, I.; Iida, T.; Sugano, Y.; Ojima, A.; Ogasawara, M.; Spaide, R.F. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010, 117, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Mitamura, Y.; Mori, T.; Akaiwa, K.; Semba, K.; Egawa, M.; Mori, J.; Sonoda, S.; Sakamoto, T. Changes in Choroidal Structures in Eyes with Chronic Central Serous Chorioretinopathy after Half-Dose Photodynamic Therapy. PLoS ONE 2016, 11, e0163104. [Google Scholar] [CrossRef] [PubMed]
- Ooto, S.; Hangai, M.; Sakamoto, A.; Tsujikawa, A.; Yamashiro, K.; Ojima, Y.; Yamada, Y.; Mukai, H.; Oshima, S.; Inoue, T.; et al. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology 2010, 117, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Fujiwara, T.; Spaide, R.F. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology 2011, 118, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.L.; Sharma, S.; Stinnett, S.; Asrani, S.; Mruthyunjaya, P. Identification of posterior segment pathology with en face retinal imaging using multicolor confocal scanning laser ophthalmoscopy. Retina 2019, 39, 972–979. [Google Scholar] [CrossRef]
- Ometto, G.; Montesano, G.; Sadeghi Afgeh, S.; Lazaridis, G.; Liu, X.; Keane, P.A.; Crabb, D.P.; Denniston, A.K. Merging Information From Infrared and Autofluorescence Fundus Images for Monitoring of Chorioretinal Atrophic Lesions. Transl. Vis. Sci. Technol. 2020, 9, 3. [Google Scholar] [CrossRef]
- Remky, A.; Arend, O.; Toonen, F. Infrared imaging of central serous chorioretinopathy: A follow-up study. Acta Ophthalmol. Scand. 1998, 76, 339–342. [Google Scholar] [CrossRef]
- Fujita, K.; Shinoda, K.; Imamura, Y.; Matsumoto, C.S.; Mizutani, Y.; Mizota, A.; Yuzawa, M. Correlation of integrity of cone outer segment tips line with retinal sensitivity after half-dose photodynamic therapy for chronic central serous chorioretinopathy. Am. J. Ophthalmol. 2012, 154, 579–585. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhu, E.Y.; Yuan, Y.; Wang, W.; Xu, G. A two-year study of diffused retinal pigment epitheliopathy treated with half-dose photodynamic therapy guided by simultaneous angiography and optical coherence tomography. Eye 2019, 33, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gal-Or, O.; Priel, E.; Rosenblatt, I.; Shulman, S.; Kramer, M. Multimodal Imaging in an Unusual Cluster of Multiple Evanescent White Dot Syndrome. J. Ophthalmol. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Median age, years (range) | 53.6 (31–84) |
| Gender (male/female) | 122/18 |
| Median disease duration months | 33.6 (2–164) |
| Current smoking | 15 (10.7%) |
| Steroid use | 34 (24.2%) |
| Drusen | 26 (18.6%) |
| Pigment epithelial detachment | 51 (36.4%) |
| FA leakage type | |
| Smokestack type | 93 (66.4%) |
| Diffuse type | 47 (33.6%) |
| Choroidal vascular hyperpermeability | 130 (92.9%) |
| Pre-hPDT | 1 Month | 3 Months | |
|---|---|---|---|
| Visual acuity (ETDRS; letters) | 77.8 ± 11.9 | 80.5 ± 11.0 | 81.5 ± 12.9 |
| Dry macula rate | 0% | 78.6% | 85% |
| CCT (µm) | 377.7 ± 115.3 | 328.9 ± 100.7 | 323.3 ± 103.1 |
| CRT (µm) | 343.7 ± 144.6 | 189.2 ± 65.6 | 185.4 ± 61.4 |
| Luminance of IR images | 131.6 ± 21.7 | 136.3 ± 20.1 | 138.8 ± 20.6 |
| Dry Macula at 1 Month | p-Value | Dry Macula at 3 Months | p-Value |
|---|---|---|---|
| Disease duration | 0.007 * | Disease duration | 0.332 |
| Pre-CRT | 0.138 | Pre-CRT | 0.043 * |
| Pre-CCT | 0.118 | CRT 1 month after-hPDT | 0.829 |
| Pre-visual acuity | 0.717 | Pre-CCT | 0.239 |
| Pre-luminance IR images | 0.994 | CCT 1 month after-hPDT | 0.373 |
| Sex | 0.144 | Pre-visual acuity (VA) | 0.020 * |
| Steroid use | 0.786 | VA 1 month after-hPDT | 0.021 * |
| Smoking habits | 0.399 | Pre-luminance IR images | 0.329 |
| Drusen (+) | 0.065 | 1 month after hPDT IR images | 0.502 |
| PED (+) | 0.389 | Dry macula 1 month at | 0.004 * |
| Leakage on FA (+) | 0.247 | Drusen (+) | 0.314 |
| CVH (+) | 0.643 | PED (+) | 0.630 |
| Leakage on FA (+) | 0.948 | ||
| CVH | 0.122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakatsuki, Y.; Tanaka, K.; Mori, R.; Furuya, K.; Kawamura, A.; Nakashizuka, H. Morphological Changes and Prognostic Factors before and after Photodynamic Therapy for Central Serous Chorioretinopathy. Pharmaceuticals 2021, 14, 53. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14010053
Wakatsuki Y, Tanaka K, Mori R, Furuya K, Kawamura A, Nakashizuka H. Morphological Changes and Prognostic Factors before and after Photodynamic Therapy for Central Serous Chorioretinopathy. Pharmaceuticals. 2021; 14(1):53. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14010053
Chicago/Turabian StyleWakatsuki, Yu, Koji Tanaka, Ryusaburo Mori, Koichi Furuya, Akiyuki Kawamura, and Hiroyuki Nakashizuka. 2021. "Morphological Changes and Prognostic Factors before and after Photodynamic Therapy for Central Serous Chorioretinopathy" Pharmaceuticals 14, no. 1: 53. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14010053