The Role of TRPA1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice
Abstract
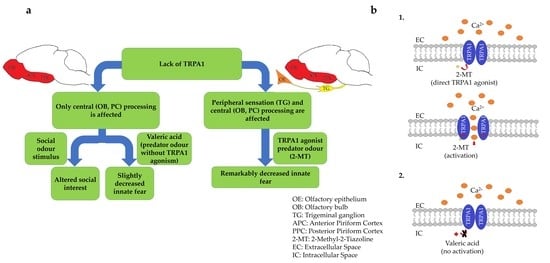
:1. Introduction
2. Results
2.1. Trpa1 mRNA Expression and Colocalisation in the Piriform Cortex, Olfactory Bulb, and in the Olfactory Epithelium by RNAscope ISH
2.2. TRPA1 and Predator Olfaction
2.2.1. Fox (2-MT) and Cat Odour (Valeric Acid) Induced Calcium Influx in Mouse and Human TRPA1-Overexpressing CHO Cells
2.2.2. Interest of Trpa1 WT and KO Animals towards Fox (2-MT) and Cat Odour (Valeric Acid)
2.2.3. Hormone (ACTH and Corticosterone) Measurements in WT and KO Mice after Using 2-MT or Valeric Acid
2.3. TRPA1 and Social Behaviour
2.3.1. Object Habituation–Dishabituation and Social Habituation–Dishabituation Tests in WT and KO Mice
2.3.2. “Three Chamber” Sociability Experiment in WT and KO Animals
2.3.3. Social Interaction and Resident–Intruder Trials in WT and KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNAscope ISH and Immunohistochemistry
4.2.1. Tissue Sample Collection and Preparation
4.2.2. RNAscope ISH on Mouse Olfactory Bulb and Piriform Cortex
4.2.3. RNAscope ISH Combined with β-Tubulin III-Immunohistochemistry
4.3. Measurement of Ca2+ Influx in TRPA1-Expressing CHO Cells in Response to 2-MT or Valeric Acid by Flow Cytometry
4.4. Behavioural Tests
4.4.1. Odour Aversion Tests and Blood Sample Collection
4.4.2. Test Battery for Social Behaviour
Day 1: Object Habituation–Dishabituation
Day 2: Sociability Test
Day 3: Social Habituation–Dishabituation
Day 4: Social Interaction
Resident–Intruder Test
4.5. Hormone Measurement
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bölcskei, K.; Kriszta, G.; Sághy, É.; Payrits, M.; Sipos, É.; Vranesics, A.; Berente, Z.; Ábrahám, H.; Ács, P.; Komoly, S.; et al. Behavioural alterations and morphological changes are attenuated by the lack of TRPA1 receptors in the cuprizone-induced demyelination model in mice. J. Neuroimmunol. 2018, 320, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sághy, É.; Sipos, É.; Ács, P.; Bölcskei, K.; Pohóczky, K.; Kemény, Á.; Sándor, Z.; Szőke, É.; Sétáló, G.J.; Komoly, S.; et al. TRPA1 deficiency is protective in cuprizone-induced demyelination—A new target against oligodendrocyte apoptosis. Glia 2016, 64, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Payrits, M.; Borbely, E.; Godo, S.; Ernszt, D.; Kemeny, A.; Kardos, J.; Szoke, E.; Pinter, E. Genetic deletion of TRPA1 receptor attenuates amyloid beta- 1-42 (Aβ(1-42))-induced neurotoxicity in the mouse basal forebrain in vivo. Mech. Ageing Dev. 2020, 189, 111268. [Google Scholar] [CrossRef] [PubMed]
- Borbély, É.; Payrits, M.; Hunyady, Á.; Mező, G.; Pintér, E. Important regulatory function of transient receptor potential ankyrin 1 receptors in age-related learning and memory alterations of mice. GeroScience 2019, 41, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.D.; Bhatnagar, K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019, 164, 17–28. [Google Scholar] [CrossRef]
- Mueller, M.; Egger, V. Dendritic integration in olfactory bulb granule cells upon simultaneous multispine activation: Low thresholds for nonlocal spiking activity. PLoS Biol. 2020, 18, e3000873. [Google Scholar] [CrossRef]
- Lage-Rupprecht, V.; Zhou, L.; Bianchini, G.; Aghvami, S.S.; Mueller, M.; Rózsa, B.; Sassoè-Pognetto, M.; Egger, V. Presynaptic NMDARs cooperate with local spikes toward GABA release from the reciprocal olfactory bulb granule cell spine. eLife 2020, 9, e63737. [Google Scholar] [CrossRef]
- Truex, R.C.; Carpenter, M.B. Human Neuroanatomy, 6th ed.; Williams and Wilkins: Baltimore, MD, USA, 1969. [Google Scholar]
- Mohrhardt, J.; Nagel, M.; Fleck, D.; Ben-Shaul, Y.; Spehr, M. Signal Detection and Coding in the Accessory Olfactory System. Chem. Senses 2018, 43, 667–695. [Google Scholar] [CrossRef] [Green Version]
- Rotermund, N.; Schulz, K.; Hirnet, D.; Lohr, C. Purinergic Signaling in the Vertebrate Olfactory System. Front. Cell. Neurosci. 2019, 13, 112. [Google Scholar] [CrossRef]
- Boesveldt, S.; Parma, V. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021, 383, 559–567. [Google Scholar] [CrossRef]
- Zou, J.; Wang, W.; Pan, Y.-W.; Lu, S.; Xia, Z. Methods to measure olfactory behavior in mice. Curr. Protoc. Toxicol. 2015, 63, 11.18.1–11.18.21. [Google Scholar] [CrossRef]
- Stoop, R. Sniffing and Oxytocin: Effects on Olfactory Memories. Neuron 2016, 90, 431–433. [Google Scholar] [CrossRef]
- Oettl, L.-L.; Kelsch, W. Oxytocin and Olfaction. Curr. Top. Behav. Neurosci. 2018, 35, 55–75. [Google Scholar] [CrossRef]
- Vosshall, L.B. Social signals: The secret language of mice. Curr. Biol. 2005, 15, R255–R257. [Google Scholar] [CrossRef] [Green Version]
- Wesson, D.W. Sniffing behavior communicates social hierarchy. Curr. Biol. 2013, 23, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Wesson, D.W.; Donahou, T.N.; Johnson, M.O.; Wachowiak, M. Sniffing behavior of mice during performance in odor-guided tasks. Chem. Senses 2008, 33, 581–596. [Google Scholar] [CrossRef] [Green Version]
- Camats Perna, J.; Engelmann, M. Recognizing Others: Rodent’s Social Memories. Curr. Top. Behav. Neurosci. 2017, 30, 25–45. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Ennaceur, A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Bleymehl, K.; Stein, B.; Pyrski, M.; Birnbaumer, L.; Munger, S.D.; Leinders-Zufall, T.; Zufall, F.; Chamero, P. Innate Predator Odor Aversion Driven by Parallel Olfactory Subsystems that Converge in the Ventromedial Hypothalamus. Curr. Biol. 2015, 25, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, M.; Imada, M.; Aizawa, S.; Sato, T. Habitat odor can alleviate innate stress responses in mice. Brain Res. 2016, 1631, 46–52. [Google Scholar] [CrossRef]
- Hacquemand, R.; Choffat, N.; Jacquot, L.; Brand, G. Comparison between low doses of TMT and cat odor exposure in anxiety- and fear-related behaviors in mice. Behav. Brain Res. 2013, 238, 227–231. [Google Scholar] [CrossRef]
- Turetsky, B.I.; Hahn, C.-G.; Borgmann-Winter, K.; Moberg, P.J. Scents and nonsense: Olfactory dysfunction in schizophrenia. Schizophr. Bull. 2009, 35, 1117–1131. [Google Scholar] [CrossRef] [Green Version]
- Hardy, C.; Rosedale, M.; Messinger, J.W.; Kleinhaus, K.; Aujero, N.; Silva, H.; Goetz, R.R.; Goetz, D.; Harkavy-Friedman, J.; Malaspina, D. Olfactory acuity is associated with mood and function in a pilot study of stable bipolar disorder patients. Bipolar Disord. 2012, 14, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Negoias, S.; Croy, I.; Gerber, J.; Puschmann, S.; Petrowski, K.; Joraschky, P.; Hummel, T. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience 2010, 169, 415–421. [Google Scholar] [CrossRef]
- Croy, I.; Schellong, J.; Joraschky, P.; Hummel, T. PTSD, but not childhood maltreatment, modifies responses to unpleasant odors. Int. J. Psychophysiol. 2010, 75, 326–331. [Google Scholar] [CrossRef]
- Rahayel, S.; Frasnelli, J.; Joubert, S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: A meta-analysis. Behav. Brain Res. 2012, 231, 60–74. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Wszolek, Z.K.; Graff-Radford, N.R.; Cookson, N.; Dickson, D.W. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol. Appl. Neurobiol. 2003, 29, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Zufall, F. TRPs in olfaction. Handb. Exp. Pharmacol. 2014, 223, 917–933. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. TRPA1 Channel as a Regulator of Neurogenic Inflammation and Pain: Structure, Function, Role in Pathophysiology, and Therapeutic Potential of Ligands. Biochemistry 2019, 84, 101–118. [Google Scholar] [CrossRef]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.; Hukkanen, M.; Saarnilehto, M.; Chapman, H.; Kuokkanen, K.; Wei, H.; Viisanen, H.; Akerman, K.E.; Lindstedt, K.; Pertovaara, A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: Sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol. Res. 2012, 65, 149–158. [Google Scholar] [CrossRef]
- Wei, H.; Hämäläinen, M.M.; Saarnilehto, M.; Koivisto, A.; Pertovaara, A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology 2009, 111, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.-C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M.O. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, F.d.V.; Villarinho, J.G.; Silva, C.R.; Oliveira, S.M.; Pinheiro, K.d.V.; Petri, D.; Rossato, M.F.; Guerra, G.P.; Trevisan, G.; Antonello Rubin, M.; et al. The involvement of the TRPA1 receptor in a mouse model of sympathetically maintained neuropathic pain. Eur. J. Pharmacol. 2015, 747, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cao, L.; Lee, C.-Y.; Matsuo, T.; Wu, K.; Asher, G.; Tang, L.; Saitoh, T.; Russell, J.; Klewe-Nebenius, D.; et al. Large-scale forward genetics screening identifies Trpa1 as a chemosensor for predator odor-evoked innate fear behaviors. Nat. Commun. 2018, 9, 2041. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Bland, K.P. Tom-cat odour and other pheromones in feline reproduction. Vet. Sci. Commun. 1979, 3, 125–136. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nishimura, T.; Yamashita, T.; Miyazaki, M. Olfactory discrimination of anal sac secretions in the domestic cat and the chemical profiles of the volatile compounds. J. Ethol. 2018, 36, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Lee, V.M.; Pixley, S.K. Age and differentiation-related differences in neuron-specific tubulin immunostaining of olfactory sensory neurons. Brain Res. Dev. Brain Res. 1994, 83, 209–215. [Google Scholar] [CrossRef]
- Packard, A.I.; Lin, B.; Schwob, J.E. Sox2 and Pax6 Play Counteracting Roles in Regulating Neurogenesis within the Murine Olfactory Epithelium. PLoS ONE 2016, 11, e0155167. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Gilbertson, M.W.; Orr, S.P.; Herzallah, M.M.; Servatius, R.J.; Myers, C.E. A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn. 2013, 81, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-I.; Lin, H.-C.; Lee, H.-T.; Tsai, F.-C.; Lee, T.-S. Loss of Transient Receptor Potential Ankyrin 1 Channel Deregulates Emotion, Learning and Memory, Cognition, and Social Behavior in Mice. Mol. Neurobiol. 2017, 54, 3606–3617. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Kondoh, K.; Lu, Z.; Ye, X.; Olson, D.P.; Lowell, B.B.; Buck, L.B. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature 2016, 532, 103–106. [Google Scholar] [CrossRef]
- White-Welkley, J.E.; Bunnell, B.N.; Mougey, E.H.; Meyerhoff, J.L.; Dishman, R.K. Treadmill exercise training and estradiol differentially modulate hypothalamic-pituitary-adrenal cortical responses to acute running and immobilization. Physiol. Behav. 1995, 57, 533–540. [Google Scholar] [CrossRef]
- Toth, M.; Mikics, E.; Tulogdi, A.; Aliczki, M.; Haller, J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm. Behav. 2011, 60, 28–36. [Google Scholar] [CrossRef]
- Koike, K.; Yoo, S.-J.; Bleymehl, K.; Omura, M.; Zapiec, B.; Pyrski, M.; Blum, T.; Khan, M.; Bai, Z.; Leinders-Zufall, T.; et al. Danger perception and stress response through an olfactory sensor for the bacterial metabolite hydrogen sulfide. Neuron 2021, 109, 2469–2484.e7. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Akopian, A.N.; Hargreaves, K.M. NGF up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Jain, P.; Li Puma, S.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.-W.; Davis, J.C.; Ding, S.; Nai, Q.; Zhou, F.-M.; Ennis, M. Expression of transient receptor potential (TRP) channel mRNAs in the mouse olfactory bulb. Neurosci. Lett. 2012, 524, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, Z.; Chen, J.; Manyande, A.; Haddad, R.; Liu, Q.; Xu, F. Cell-Type-Specific Whole-Brain Direct Inputs to the Anterior and Posterior Piriform Cortex. Front. Neural Circuits 2020, 14, 4. [Google Scholar] [CrossRef]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2011, 15, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Ma, D.; Grubb, B.D.; Wang, M. ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J. Headache Pain 2019, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, N.B.; Kolodziejczyk, K.; Kougioumtzidou, E.; Attwell, D. Proton-gated Ca(2+)-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 2016, 529, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Bosson, A.; Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A.; Albrieux, M. TRPA1 channels promote astrocytic Ca(2+) hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-β peptide. Mol. Neurodegener. 2017, 12, 53. [Google Scholar] [CrossRef]
- Marin, C.; Laxe, S.; Langdon, C.; Alobid, I.; Berenguer, J.; Fuentes, M.; Bernabeu, M.; Mullol, J. Olfactory Training Prevents Olfactory Dysfunction Induced by Bulbar Excitotoxic Lesions: Role of Neurogenesis and Dopaminergic Interneurons. Mol. Neurobiol. 2019, 56, 8063–8075. [Google Scholar] [CrossRef]
- Sairazi, N.S.M.; Sirajudeen, K.N.S.; Asari, M.A.; Mummedy, S.; Muzaimi, M.; Sulaiman, S.A. Effect of tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complement. Altern. Med. 2017, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Wu, A.; Berglund, K.; Gu, X.; Jiang, M.Q.; Talati, J.; Zhao, J.; Wei, L.; Yu, S.P. Pathogenesis of sporadic Alzheimer’s disease by deficiency of NMDA receptor subunit GluN3A. Alzheimer’s Dement. 2021. [Google Scholar] [CrossRef]
- Meza, N.W.; Puyet, A.; Pérez-Benavente, S.; Quintana-Bustamante, O.; Diez, A.; Bueren, J.A.; Segovia, J.C.; Bautista, J.M. Functional analysis of gammaretroviral vector transduction by quantitative PCR. J. Gene Med. 2006, 8, 1097–1104. [Google Scholar] [CrossRef]
- Nemes, B.; Bölcskei, K.; Kecskés, A.; Kormos, V.; Gaszner, B.; Aczél, T.; Hegedüs, D.; Pintér, E.; Helyes, Z.; Sándor, Z. Human Somatostatin SST(4) Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization. Int. J. Mol. Sci. 2021, 22, 3758. [Google Scholar] [CrossRef]
- Pozsgai, G.; Payrits, M.; Sághy, É.; Sebestyén-Bátai, R.; Steen, E.; Szőke, É.; Sándor, Z.; Solymár, M.; Garami, A.; Orvos, P.; et al. Analgesic effect of dimethyl trisulfide in mice is mediated by TRPA1 and sst(4) receptors. Nitric Oxide Biol. Chem. 2017, 65, 10–21. [Google Scholar] [CrossRef]
- Zelena, D.; Mergl, Z.; Foldes, A.; Kovács, K.J.; Tóth, Z.; Makara, G.B. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1110–E1117. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, J.; Wei, X.; Liao, J.; Xu, Y.; Lu, T.; Qin, B.; Xie, J.; Deng, C.; Huang, X. Alterations of NMDA receptor binding in various brain regions among 6-hydroxydopamine-induced Parkinsonian rats. Int. J. Neurosci. 2014, 124, 457–465. [Google Scholar] [CrossRef]
- Vaughan, D.N.; Jackson, G.D. The piriform cortex and human focal epilepsy. Front. Neurol. 2014, 5, 259. [Google Scholar] [CrossRef] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konkoly, J.; Kormos, V.; Gaszner, B.; Sándor, Z.; Kecskés, A.; Alomari, A.; Szilágyi, A.; Szilágyi, B.; Zelena, D.; Pintér, E. The Role of TRPA1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice. Pharmaceuticals 2021, 14, 1336. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14121336
Konkoly J, Kormos V, Gaszner B, Sándor Z, Kecskés A, Alomari A, Szilágyi A, Szilágyi B, Zelena D, Pintér E. The Role of TRPA1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice. Pharmaceuticals. 2021; 14(12):1336. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14121336
Chicago/Turabian StyleKonkoly, János, Viktória Kormos, Balázs Gaszner, Zoltán Sándor, Angéla Kecskés, Ammar Alomari, Alíz Szilágyi, Beatrix Szilágyi, Dóra Zelena, and Erika Pintér. 2021. "The Role of TRPA1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice" Pharmaceuticals 14, no. 12: 1336. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14121336