Evaluation of the Antiviral Activity of Sitagliptin-Glatiramer Acetate Nano-Conjugates against SARS-CoV-2 Virus
Abstract
:1. Introduction
2. Results
2.1. Experimental Design of SIT-GA Nano-Conjugates
2.1.1. Analysis of the Factorial Design
2.1.2. Effect of Variables on Particle Size (Y1)
2.1.3. Effect of Variables on the Zeta Potential (Y2)
2.2. Selection of the Optimized SIT-GA Nano-Conjugates
2.3. Fourier-Transform Infrared Spectroscopy Investigation of the Optimized SIT-GA Nano-Complex
2.4. Transmission Electron Microscope (TEM) Investigation of the Optimized SIT-GA Nano-Conjugates
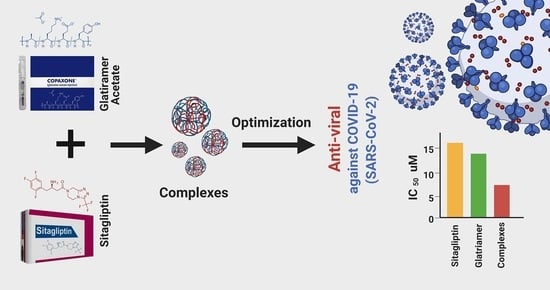
2.5. In Vitro Antiviral Screening Activity
2.6. In Vitro Mpro, 3CL Protease Inhibition Test
2.7. Molecular Docking and Virtual Screening Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design for the Preparation and Optimization of SIT-GA Nano-Conjugates
4.2.1. Preparation of the SIT-GA Formulations
4.2.2. Determination of the Particle Size and Zeta Potential
4.2.3. Optimization of the SIT-GA Preparations
4.2.4. FTIR Spectroscopy Investigation of the Optimized SIT-GA Complex
4.2.5. TEM Investigation of the Optimized SIT-GA Nano-Conjugates
4.3. Determination of the CC50 and IC50 Values
4.4. In Vitro 3CL Protease Inhibition Test
4.5. Docking Studies
4.5.1. Optimization of Target Compounds
4.5.2. Docking of the Target Molecules to the Active Binding Site of the Crystallographic Structure of Mpro (PDB ID: 6LU7)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vepa, A.; Bae, J.P.; Ahmed, F.; Pareek, M.; Khunti, K. COVID-19 and ethnicity: A novel pathophysiological role for inflammation. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.; Baric, R.; Groot, R.J.D.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of sars-cov-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Marraro, G.A.; Zeng, Y. From SARS and MERS to COVID-19: A brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Valencia, I.; Peiró, C.; Lorenzo, Ó.; Sánchez-Ferrer, C.F.; Eckel, J.; Romacho, T. DPP4 and ACE2 in Diabetes and COVID-19: Therapeutic Targets for Cardiovascular Complications? Front. Pharmacol. 2020, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mazucanti, C.H.; Egan, J.M. Sars-cov-2 disease severity and diabetes: Why the connection and what is to be done? Immun. Ageing 2020, 17, 21. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Wong, J.; Henry, B.M. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): A pooled analysis. Pol. Arch. Intern. Med. 2020, 130, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.H. An overview of severe acute respiratory syndrome-coronavirus (sars-cov) 3cl protease inhibitors: Peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Hajjo, R.; Sabbah, D.A. Sitagliptin: A potential drug for the treatment of COVID-19? Acta Pharm. 2021, 71, 175–184. [Google Scholar] [CrossRef]
- Solerte, S.B.; D’Addio, F.; Trevisan, R.; Lovati, E.; Rossi, A.; Pastore, I.; Dell’Acqua, M.; Ippolito, E.; Scaranna, C.; Bellante, R.; et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated With Reduced Mortality in Patients With Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care 2020, 43, 2999–3006. [Google Scholar] [CrossRef]
- Ugwueze, C.V.; Ezeokpo, B.C.; Nnolim, B.I.; Agim, E.A.; Anikpo, N.C.; Onyekachi, K.E. COVID-19 and Diabetes Mellitus: The Link and Clinical Implications. Dubai Diabetes Endocrinol. J. 2020, 26, 69–77. [Google Scholar] [CrossRef]
- Lambeir, A.-M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-Peptidase IV from Bench to Bedside: An Update on Structural Properties, Functions, and Clinical Aspects of the Enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef] [PubMed]
- Röhrborn, D.; Eckel, J.; Sell, H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 2014, 588, 3870–3877. [Google Scholar] [CrossRef] [Green Version]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.A.; Kim, E.; Moodie, J. Glatiramer in the treatment of multiple sclerosis. Int. J. Nanomed. 2006, 1, 283–289. [Google Scholar]
- Berger, J.R.; Brandstadter, R.; Bar-Or, A. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e761. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, M.; Elemam, N.M.; Hundt, J.E.; Maghazachi, A.A. Drugs for Multiple Sclerosis Activate Natural Killer Cells: Do They Protect Against COVID-19 Infection? Infect. Drug Resist. 2020, 13, 3243–3254. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Awan, Z.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Ibrahim, T.S.; Asfour, H.Z.; Al-Rabia, M.W.; Alfarsi, A.; Alhakamy, N.A.; Abdulaal, W.H.; Al Al Sadoun, H.; et al. The Enhanced Cytotoxic and Pro-Apoptotic Effects of Optimized Simvastatin-Loaded Emulsomes on MCF-7 Breast Cancer Cells. Pharmaceutics 2020, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, U.A.; Aldawsari, H.M.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Alhakamy, N.A.; Alsulimani, H.H.; Caraci, F.; Caruso, G. The Encapsulation of Febuxostat into Emulsomes Strongly Enhances the Cytotoxic Potential of the Drug on HCT 116 Colon Cancer Cells. Pharmaceutics 2020, 12, 956. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.; Aldawsari, H. Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; et al. Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics 2020, 12, 346. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Al-Saleem, M.S.M.; Ahmed, O.A.A.; Fahmy, U.A.; Alhakamy, N.A.; Eid, B.G.; Abdel-Naim, A.B.; Abdel-Mageed, W.M.; Alrasheed, M.M.; Shazly, G.A. Optimized Conjugation of Fluvastatin to HIV-1 TAT Displays Enhanced Pro-Apoptotic Activity in HepG2 Cells. Int. J. Mol. Sci. 2020, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Hogard, M.L.; Caruso, G.; Costa, E.E.M.; Lazzarino, G.; Lunte, S.M. Monitoring carnosine uptake by RAW 264.7 macrophage cells using microchip electrophoresis with fluorescence detection. Anal. Methods 2017, 9, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Jeynes, J.C.G.; Jeynes, C.; Merchant, M.J.; Kirkby, K.J. Measuring and modelling cell-to-cell variation in uptake of gold nanoparticles. Analyst 2013, 138, 7070. [Google Scholar] [CrossRef] [Green Version]
- Khetan, J.; Shahinuzzaman, M.; Barua, S.; Barua, D. Quantitative Analysis of the Correlation between Cell Size and Cellular Uptake of Particles. Biophys. J. 2019, 116, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S.; Hami, I.; Sawrav, M.S.S.; Rabbi, M.F.; Saha, O.; Bahadur, N.M.; Rahaman, M.M. Drug repurposing for prevention and treatment of covid-19: A clinical landscape. Discoveries 2020, 8, e121. [Google Scholar] [CrossRef]
- Mostafa, A.; Kandeil, A.; Elshaier, Y.A.M.M.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceutics 2020, 13, 443. [Google Scholar] [CrossRef]
- DeRosa, G.; D’Angelo, A.; Maffioli, P. Sitagliptin in type 2 diabetes mellitus: Efficacy after five years of therapy. Pharmacol. Res. 2015, 100, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, N.; Korenstein, R. The uptake of HIV Tat peptide proceeds via two pathways which differ from macropinocytosis. Biochim. Biophys. Acta 2015, 1848, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Peng, Q.; Wang, P.; Zhou, B. Progress in Research and Application of HIV-1 TAT-Derived Cell-Penetrating Peptide. J. Membr. Biol. 2017, 250, 115–122. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Clavadetscher, J.; Lee, D.-K.; Chankeshwara, S.V.; Bradley, M.; Cho, W.-S. Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials 2018, 8, 1028. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- He, J.; Hu, L.; Huang, X.; Wang, C.; Zhang, Z.; Wang, Y.; Zhang, D.; Ye, W. Potential of coronavirus 3c-like protease inhibitors for the development of new anti-sars-cov-2 drugs: Insights from structures of protease and inhibitors. Int. J. Antimicrob. Agents 2020, 56, 106055. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Mostafa, A.; El-Shesheny, R.; Shehata, M.; Roshdy, W.H.; Ahmed, S.S.; Gomaa, M.; Taweel, A.E.; Kayed, A.E.; Mahmoud, S.H.; et al. Coding-complete genome sequences of two sars-cov-2 isolates from egypt. Microbiol. Resour. Announc. 2020, 9, e00489-20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; George, S.; Schmidt, M.F.; Al-Gharabli, S.I.; Rademann, J.; Hilgenfeld, R. Peptide aldehyde inhibitors challenge the substrate specificity of the SARS-coronavirus main protease. Antivir. Res. 2011, 92, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Ibrahim, T.S.; Salem, I.M.; Salama, I.; Khayyat, A.N.; Mostafa, S.M.; El-Sabbagh, O.I.; Darwish, K.M. The Anticancer Activity for the Bumetanide-Based Analogs via Targeting the Tumor-Associated Membrane-Bound Human Carbonic Anhydrase-IX Enzyme. Pharmaceutics 2020, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Mestres-Truyol, J.; Ojeda-Montes, M.J.; Macip, G.; Saldivar-Espinoza, B.; Cereto-Massagué, A.; Pujadas, G.; Garcia-Vallvé, S. Prediction of novel inhibitors of the main protease (m-pro) of sars-cov-2 through consensus docking and drug reposition. Int. J. Mol. Sci. 2020, 21, 3793. [Google Scholar] [CrossRef]









| Experimental Run Number | Independent Variables | Particle Size ± S.D. | Zeta Potential ± S.D. | ||
|---|---|---|---|---|---|
| SIT Concentration (mM) | GA Concentration (mM) | pH | |||
| F-1 | 1 | 10 | 6 | 220.8 ± 3.6 | 18.7 ± 0.2 |
| F-2 | 10 | 1 | 10 | 147.8 ± 1.2 | 32.2 ± 0.9 |
| F-3 | 1 | 1 | 6 | 77.4 ± 0.9 | 7.3 ± 0.1 |
| F-4 | 10 | 10 | 10 | 247.7 ± 2.9 | 29.1 ± 0.6 |
| F-5 | 10 | 10 | 6 | 276.5 ± 3.1 | 9.4 ± 0.2 |
| F-6 | 1 | 10 | 10 | 206.7 ± 2.6 | 33.3 ± 0.7 |
| F-7 | 10 | 1 | 6 | 136.3 ± 1.6 | 6.2 ± 0.1 |
| F-8 | 1 | 1 | 10 | 78.7 ± 1.0 | 27.1 ± 1.1 |
| Responses | Process Order | p-Value | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Factors and Interactions |
|---|---|---|---|---|---|---|---|
| Y1: particle size (nm) | Main effects | 0.0004 | 0.9851 | 0.9740 | 0.9405 | 22.28 | X1, X2 |
| Y2: zeta potential (mV) | 2FI | 0.0227 | 0.9999 | 0.9990 | 0.9906 | 77.86 | X2, X3, X1X2 |
| Variables | X1: SIT Concentration (mM) | X2: GA Concentration (mM) | X3: Hydrating Buffer pH |
|---|---|---|---|
| Optimum values | 1.0 | 1.0 | 10 |
| Predicted value | Observed value | Error % | |
| Particle size (nm) | 78.24 | 77.42 | 1.06 |
| Zeta potential (mV) | 27.17 | 27.67 | 1.84 |
| Independent Variables | Levels | |
|---|---|---|
| (‒1) | (+1) | |
| X1: SIT concentration (mM) | 1 | 10 |
| X2: GA concentration (mM) | 1 | 10 |
| X3: pH | 6 | 10 |
| Responses | Desirability constraints | |
| Y1: particle size (nm) | Minimize | |
| Y2: zeta potential (mV) | Maximize | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; Ahmed, O.A.A.; Ibrahim, T.S.; Aldawsari, H.M.; Eljaaly, K.; Fahmy, U.A.; Alaofi, A.L.; Caraci, F.; Caruso, G. Evaluation of the Antiviral Activity of Sitagliptin-Glatiramer Acetate Nano-Conjugates against SARS-CoV-2 Virus. Pharmaceuticals 2021, 14, 178. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14030178
Alhakamy NA, Ahmed OAA, Ibrahim TS, Aldawsari HM, Eljaaly K, Fahmy UA, Alaofi AL, Caraci F, Caruso G. Evaluation of the Antiviral Activity of Sitagliptin-Glatiramer Acetate Nano-Conjugates against SARS-CoV-2 Virus. Pharmaceuticals. 2021; 14(3):178. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14030178
Chicago/Turabian StyleAlhakamy, Nabil A., Osama A. A. Ahmed, Tarek S. Ibrahim, Hibah M. Aldawsari, Khalid Eljaaly, Usama A. Fahmy, Ahmed L. Alaofi, Filippo Caraci, and Giuseppe Caruso. 2021. "Evaluation of the Antiviral Activity of Sitagliptin-Glatiramer Acetate Nano-Conjugates against SARS-CoV-2 Virus" Pharmaceuticals 14, no. 3: 178. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14030178