Comprehensive and Rapid Quality Evaluation Method for the Ayurvedic Medicine Divya-Swasari-Vati Using Two Analytical Techniques: UPLC/QToF MS and HPLC–DAD
Abstract
:1. Introduction
2. Results
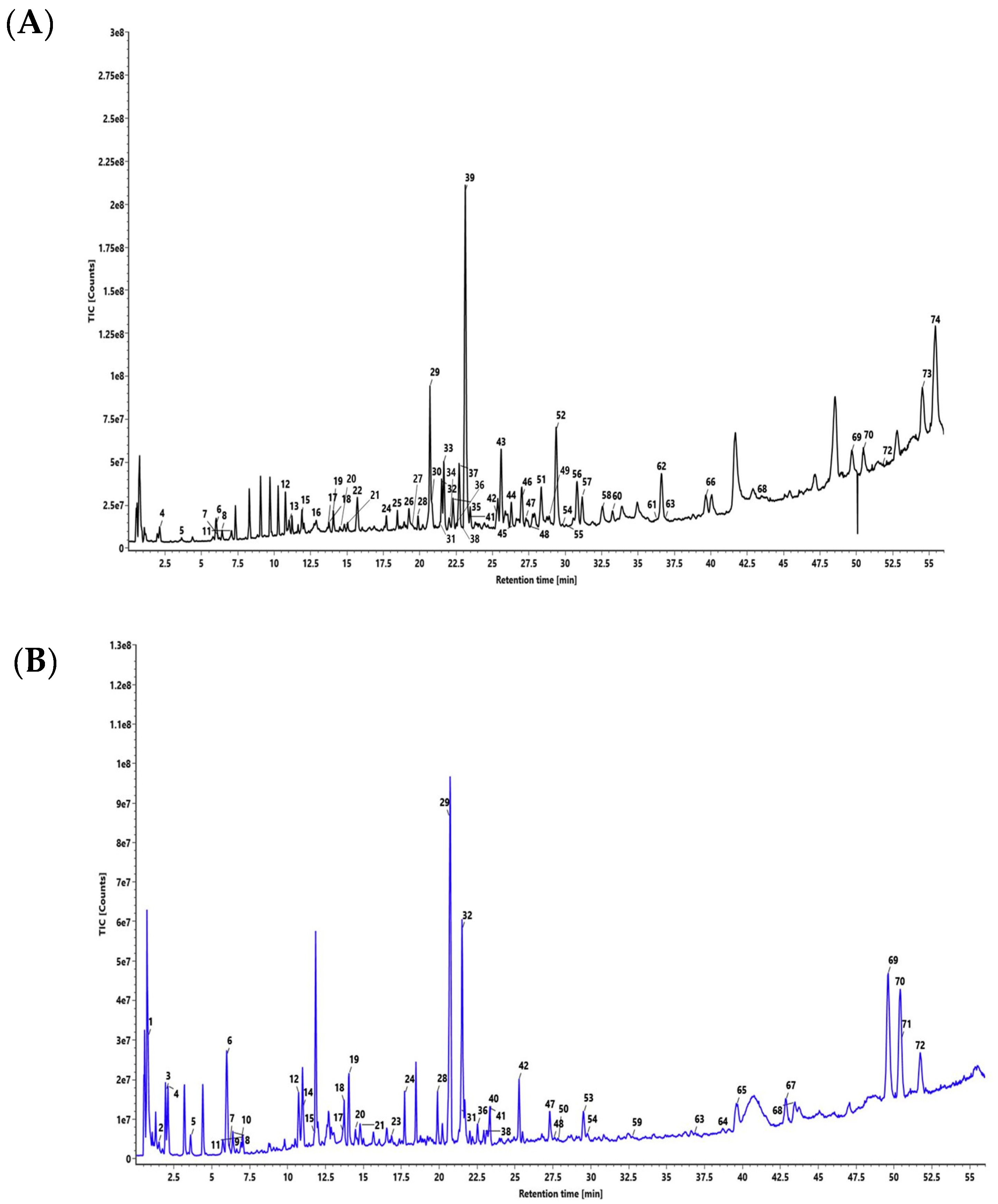
2.1. UPLC/QToF MS Analysis Characterized Chemical Markers in DSV
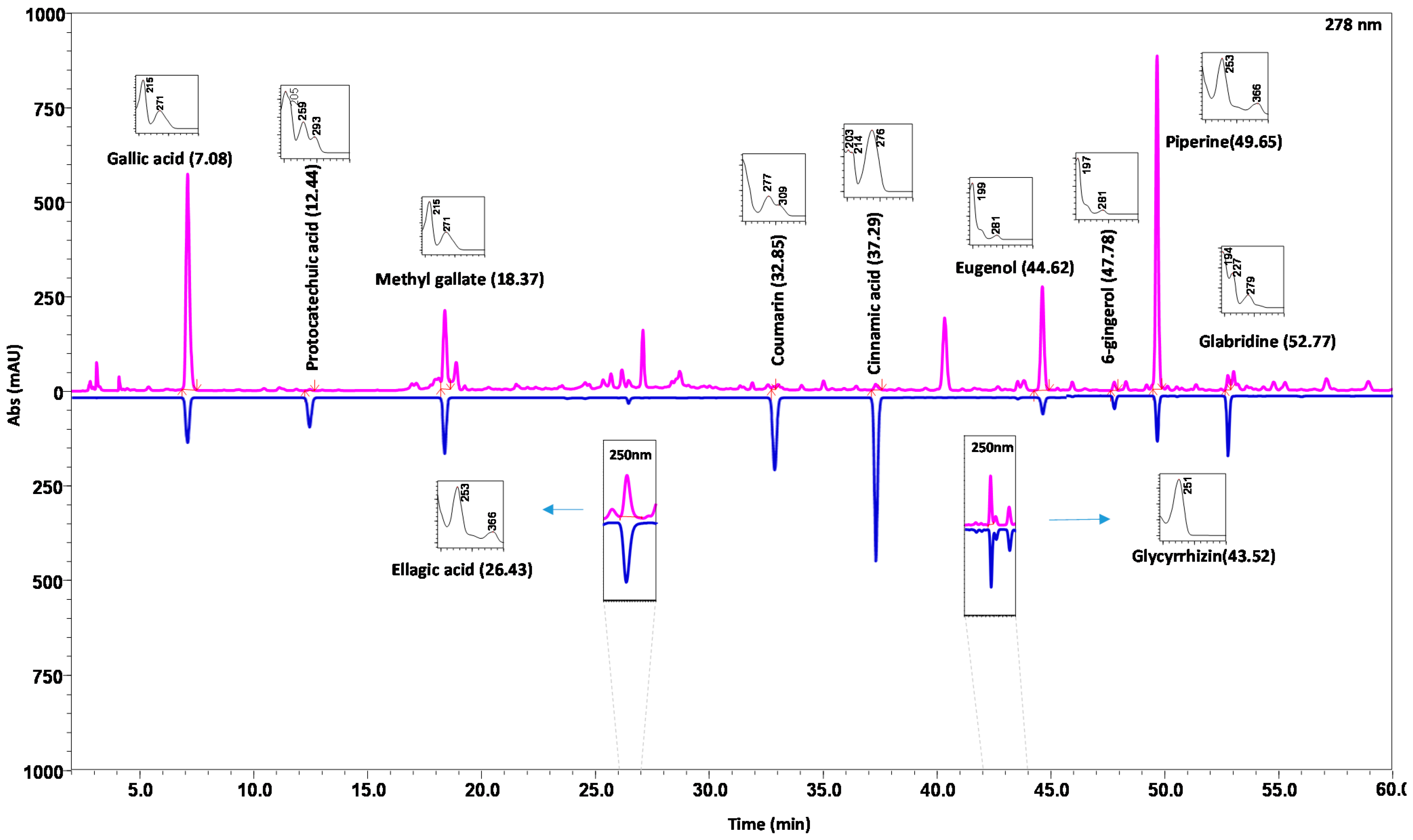
2.2. Establishment and Optimization of the HPLC–DAD Method:
2.3. Validation of the Developed and Optimized HPLC Method for Quantitative Analysis of Eleven Marker components in DSV
2.3.1. Specificity, Linearity, Limits of Quantification and Detection
2.3.2. Accuracy and Precision
2.3.3. Robustness and Ruggedness
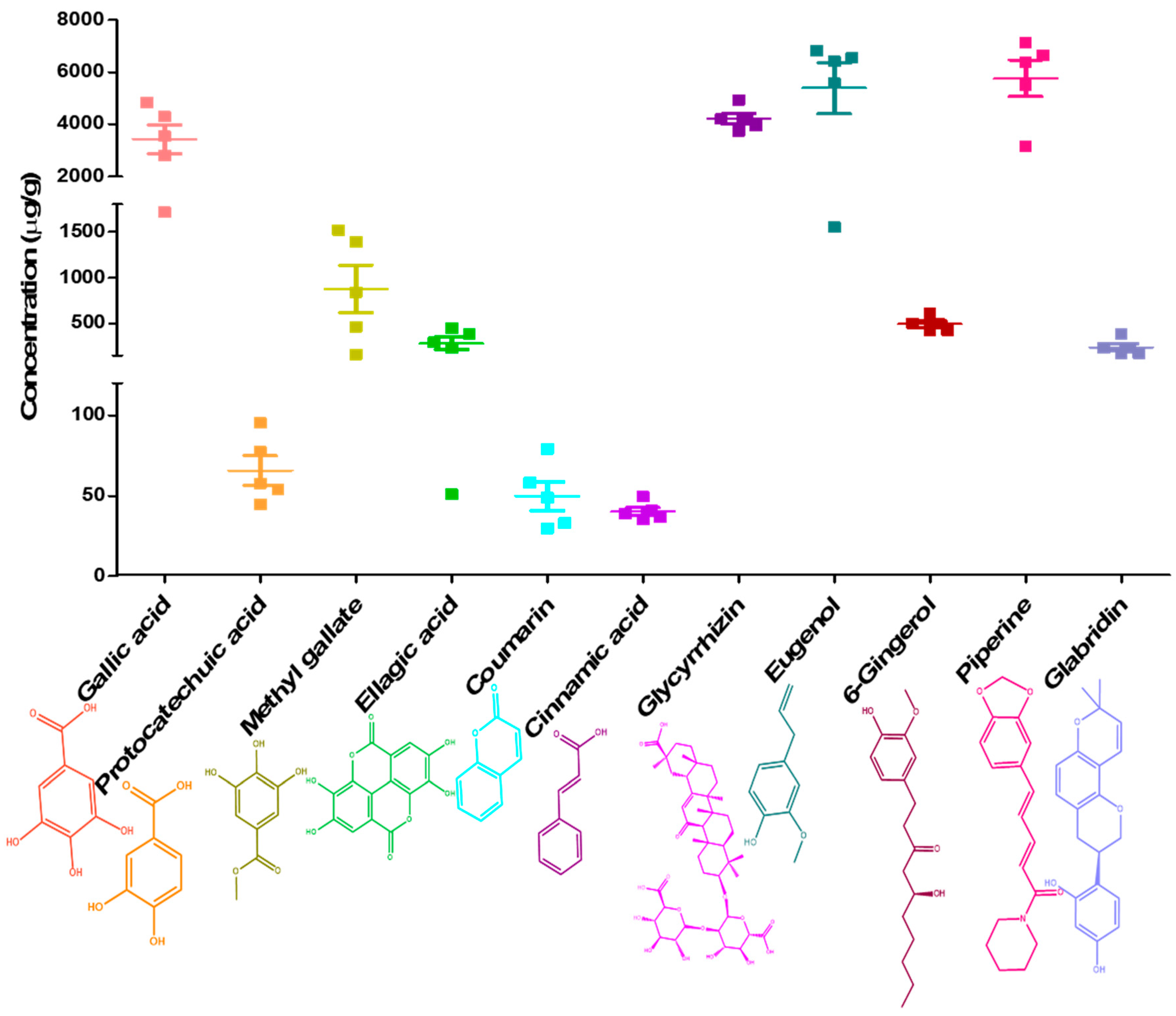
2.4. Validated HPLC–DAD Method Simultaneously Quantified Eleven Marker Analytes in Five Different Batches of DSV
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Samples
4.2. Analytical Investigations
4.2.1. UPLC/QToF MS Analysis
- Preparation of DSV sample solution:
- Instrumentation
- Identification of marker components in DSV
4.2.2. HPLC–DAD Method Development and Optimization
- Preparation of standard solution:
- Preparation of DSV sample solution
- Instrumentation and chromatographic conditions
4.3. Method Validation
4.4. Quantitative Analysis of Targeted Analytes in Five Different Batches of DSV
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef]
- Casadei, E.; Salinas, I. Comparative models for human nasal infections and immunity. Dev. Comp. Immunol. 2019, 92, 212–222. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, S.; Ghangrekar, M.M. The COVID-19 pandemic: Biological evolution, treatment options and consequences. Innov. Infrastruct. Solut. 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Antonio, A.D.S.; Wiedemann, L.S.M.; Veiga-Junior, V.F. Natural products’ role against COVID-19. RSC Adv. 2020, 10, 23379–23393. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evid. Based Complement Alternat. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.; Jiang, Y.; Kao, R.Y.; Lu, H.; Fan, K.; Cheng, V.C.; Tsui, W.H.; Hung, I.F.; Lee, T.S. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Rana, S.; Shahzad, M.; Shabbir, A. Pistacia integerrima ameliorates airway inflammation by attenuation of TNF-α, IL-4, and IL-5 expression levels, and pulmonary edema by elevation of AQP1 and AQP5 expression levels in mouse model of ovalbumin-induced allergic asthma. Phytomedicine 2016, 23, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Priyashree, S.; Jha, S.; Pattanayak, S.P. Bronchodilatory and mast cell stabilising activity of Cressa cretica L.: Evaluation through in vivo and in vitro experimental models. Asian Pac. J. Trop. Med. 2012, 5, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Rathinavel, T.; Palanisamy, M.; Palanisamy, S.; Subramanian, A.; Thangaswamy, S. Phytochemical 6-Gingerol—A promising Drug of choice for COVID-19. Int. J. Adv. Sci. Eng. 2020, 06, 1482–1489. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.-G.; Lee, M.Y.; Lee, Y.-M.; Bae, J.-S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Balkrishna, A.; Solleti, S.K.; Singh, H.; Tomer, M.; Sharma, N.; Varshney, A. Calcio-herbal formulation, Divya-Swasari-Ras, alleviates chronic inflammation and suppresses airway remodelling in mouse model of allergic asthma by modulating pro-inflammatory cytokine response. Biomed. Pharmacother. 2020, 126, 110063. [Google Scholar] [CrossRef]
- Balkrishna, A.; Verma, S.; Solleti, S.K.; Khandrika, L.; Varshney, A. Calcio-herbal medicine Divya-Swasari-Vati ameliorates SARS-CoV-2 spike protein-induced pathological features and inflammation in humanized zebrafish model by moderating IL-6 and TNF-α cytokines. J. Inflamm. Res. 2020, 13, 1219–1243. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Cieśla, Ł. Biological fingerprinting of herbal samples by means of liquid chromatography. Chromatogr. Res. Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- WHO. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Shoko, T.; Maharaj, V.J.; Naidoo, D.; Tselanyane, M.; Nthambeleni, R.; Khorombi, E.; Apostolides, Z. Anti-aging potential of extracts from Sclerocarya birrea (A. Rich.) Hochst and its chemical profiling by UPLC-Q-TOF-MS. BMC Complement Altern. Med. 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonization (ICH-Q2 R1). Validation of Analytical Procedures: Text and Methodology; ICH Secretariat: Geneva, Switzerland, 2005.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med. 2013, 2013, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A. Phytochemistry and traditional medicine—The revolution continues. Phytochem. Lett. 2014, 10, 391–398. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Betz, J.M.; Brown, P.N.; Roman, M.C. Accuracy, precision, and reliability of chemical measurements in natural products research. Fitoterapia 2011, 82, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.-J.; Zhang, J.-Q.; Yao, C.-L.; Bauer, R.; Khan, I.A.; Wu, W.-Y.; Guo, D. Deeper chemical perceptions for better traditional chinese medicine standards. Engineering 2019, 5, 83–97. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Quality Control, Screening, Toxicity, and Regulation of Herbal Drugs. In Modern Phytomedicine; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 25–57. ISBN 3527315306. [Google Scholar]
- Zhou, Y.; Huang, S.-X.; Pu, J.-X.; Li, J.-R.; Ding, L.-S.; Chen, D.-F.; Sun, H.-D.; Xu, H.-X. Ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometric procedure for qualitative and quantitative analyses of nortriterpenoids and lignans in the genus Schisandra. J. Pharm. Biomed. Anal. 2011, 56, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Vogeser, M.; Seger, C. A decade of HPLC–MS/MS in the routine clinical laboratory—Goals for further developments. Clin. Biochem. 2008, 41, 649–662. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phyther. Res. 2018, 32, 2329–2339. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Jawhari, F.Z.; Moussaoui, A.E.L.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E.; et al. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between total phenolic and flavonoid contents with antioxidant and antimicrobial activities of chemically characterized Extracts. Plants. 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Mukherjee, K.; Mal, M.; Wahile, A.; Saha, B.P.; Mukherjee, P.K. Determination of 6-gingerol in ginger (Zingiber officinale) using high-performance thin-layer chromatography. J. Sep. Sci. 2006, 29, 2292–2295. [Google Scholar] [CrossRef]
- Zahoor, M.; Zafar, R.; Rahman, N.U. Isolation and identification of phenolic antioxidants from Pistacia integerrima gall and their anticholine esterase activities. Heliyon 2018, 4, e01007. [Google Scholar] [CrossRef] [Green Version]
- Sulekha, G.; Tambe, E. Identification of chemical constituents of cinnamon bark oil by GCMS and comparative study garnered from five different countries. Glob. J. Sci. Front. Res. C Biol. Sci. 2019, 19, 35–42. [Google Scholar]
- Pal, D.; Sahu, C.; Haldar, A. Bhasma: The ancient Indian nanomedicine. J. Adv. Pharm. Technol. Res. 2014, 5, 4–12. [Google Scholar] [CrossRef]
- Sahoo, I.; More, S.S.; Jadhav, V.; Dalai, S.; Sahoo, M. Clinical appraisal on therapeutic efficacy of tankana & sphatika bhasma with madhu pratisarana in tundikeri. J. Drug Deliv. Ther. 2019, 9, 130–134. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, A.K.; Tiwari, O.P.; Jha, S. In-house preparation and characterization of an Ayurvedic bhasma: Praval bhasma. J. Integr. Med. 2014, 12, 52–58. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.-J.E.; Komaitis, M. RP-HPLC Analysis of the phenolic compounds of plant extracts. investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Wilson, I.; Brinkman, U.A.T. Hyphenation and hypernation: The practice and prospects of multiple hyphenation. J. Chromatogr. A 2003, 1000, 325–356. [Google Scholar] [CrossRef]
- Shabir, G.A. Validation of high-performance liquid chromatography methods for pharmaceutical analysis. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Bioanalytical Method Validation Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration: Rockville, MD, USA, 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 15 March 2021).
- USP. <1225> Validation of Compendial Procedures. United States Pharmacopoeia XXXVII Natl. Formul. XXXII 2007. Available online: http://www.uspbpep.com/usp29/v29240/usp29nf24s0_c1225.html (accessed on 15 March 2021).
- Seno, S.; Ohtake, S.; Kohno, H. Analytical validation in practice at a quality control laboratory in the Japanese pharmaceutical industry. In Validation in Chemical Measurement; Springer: Berlin/Heidelberg, Germany, 1997; pp. 56–61. ISBN 3540207880. [Google Scholar]
- Prichard, L.; Barwick, V. Preparation of Calibration Curves a Guide to Best Practice; LGC Limited: Teddington, UK, 2003. [Google Scholar] [CrossRef]
| S. No. | DSV Constituent’s Scientific Name | Hindi Vernacular Name | % in Each DSV Tablet |
|---|---|---|---|
| 1 | Pistacia integerrima | Kakadasingi | 11.66 |
| 2 | Glycyrrhiza glabra | Mulethi | 11.85 |
| 3 | Cressa cretica | Rudanti | 11.66 |
| 4 | Piper nigrum | Marich | 7.77 |
| 5 | Piper longum | Choti pippal | 7.77 |
| 6 | Zingiber officinale | Sounth | 7.77 |
| 7 | Cinnamomum zeylanicum | Dalchini | 5.92 |
| 8 | Syzygium aromaticum | Lavang | 5.92 |
| 9 | Anacylus pyrethrum | Akarkara | 5.92 |
| 10 | Herbally processed ash from calcined shell of pearl oyster (Pinctada fucata) | Mukta- Shukti Bhasma | 2.33 |
| 11 | Herbally processed ash from rich gypsum | Godanti Bhasma | 2.33 |
| 12 | Herbally processed ash from calcined cowry shell of Cypraea moneta | Kapardak Bhasma | 2.33 |
| 13 | Herbally processed ash from calcined mica | Abharak Bhasma | 2.33 |
| 14 | Herbally processed ash from calcined form of alum | Sphatika Bhasma | 2.33 |
| 15 | Coral calcium powder processed with rose water | Praval Pishti | 2.33 |
| 16 | Herbally processed ash from calcined borax | Tankan Bhasma | 2.33 |
| Peak | Analyte | Formula | Neutral Mass (D) | Observed Mass (D) | RT (min) | Mode | Fragments |
|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | C7H12O6 | 192.0634 | 191.0555 | 0.80 | −ve | [C7H12O6]−H, m/z 173.0445, m/z 149.0443, m/z 129.0184, m/z 113.0258, m/z 89.0267 |
| 2 | Galloylglucose | C13H16O10 | 332.0744 | 331.0665 | 1.50 | −ve | [C13H16O10]−H, m/z 271.0442, m/z 211.0231, m/z 169.0130, m/z 151.0026 |
| 3 | Gallic acid | C7H6O5 | 170.0215 | 169.0136 | 1.95 | −ve | [C7H6O5]−H, m/z 153.0177, m/z 137.0238, m/z 125.0238 |
| 4 | Theogallin | C14H16O10 | 344.0744 | 345.0821 | 2.13 | +ve | [C14H16O10]+H, m/z 327.0714, m/z 247.0211, m/z 192.0607, m/z 153.0187, m/z 125.0239 |
| 343.0667 | 2.00 | −ve | [C14H16O10]−H, m/z 297.0600, m/z 271.0448, m/z 191.0550, m/z 166.9973, m/z 123.0092 | ||||
| 5 | Protocatechuic acid | C7H6O4 | 154.0266 | 155.0340 | 3.65 | +ve | [C7H6O4]+H, m/z 137.0237 |
| 153.0185 | 3.61 | −ve | [C7H6O4]−H | ||||
| 6 | Methyl gallate | C8H8O5 | 184.0372 | 185.0447 | 6.04 | +ve | [C8H8O5]+H, m/z 169.0107, m/z 153.0186, m/z 139.0408 |
| 183.0292 | 5.99 | −ve | [C8H8O5]−H, m/z 168.0051, m/z 153.0181, m/z 124.0160, m/z 123.0079, m/z 106.0077 | ||||
| 7 | 3,4-Di-O-galloylquinic acid | C21H20O14 | 496.0853 | 497.0923 | 6.24 | +ve | [C21H20O14]+H, m/z 327.0702, m/z 247.0232, m/z 153.0186, m/z 139.0408 |
| 495.0775 | 6.18 | −ve | [C21H20O14]−H, m/z 343.0652, m/z 245.0076, m/z 191.0547, m/z 166.9966 | ||||
| 8 | Chlorogenic acid | C16H18O9 | 354.0951 | 355.1026 | 6.43 | +ve | [C16H18O9]+H, m/z 319.0814, m/z 235.0602, m/z 205.0496, m/z 163.0395, m/z 130.0664 |
| 353.0874 | 6.39 | −ve | [C16H18O9]−H, m/z 275.0537, m/z 233.0444, m/z 205.0495, m/z 163.0388 | ||||
| 9 | 1,6-Di-O-galloyl-glucose | C20H20O14 | 484.0853 | 483.0775 | 6.64 | −ve | [C20H20O14]−H, m/z 313.0547, m/z 271.0442, m/z 169.0129, m/z 169.0050 |
| 10 | Digallic acid | C14H10O9 | 322.0325 | 321.0246 | 6.94 | −ve | [C14H10O9]−H, m/z 275.0173, m/z 257.0064, m/z 169.0130, m/z 168.0047, m/z 125.0237 |
| 11 | Cryptochlorogenic acid | C16H18O9 | 354.0951 | 355.1028 | 7.08 | +ve | [C16H18O9]+H, m/z 319.0818, m/z 301.0712, m/z 235.0606, m/z 217.0499, m/z 149.0238 |
| 353.0873 | 7.05 | −ve | [C16H18O9]−H, m/z 335.0735, m/z 233.0442, m/z 217.0489, m/z 217.0489, m/z 191.0324, m/z 147.0429 | ||||
| 12 | Neoliquiritin | C21H22O9 | 418.1264 | 419.1343 | 10.76 | +ve | [C21H22O9]+H, m/z 389.1238, m/z 285.0760, m/z 257.0813, m/z 191.0330, m/z 137.0238, m/z 133.0863 |
| 417.1192 | 10.73 | −ve | [C21H22O9]−H, m/z 399.1010, m/z 297.0736, m/z 255.0651, m/z 254.0565, m/z 191.0328, m/z 135.0079 | ||||
| 13 | Liquiritigenin | C15H12O4 | 256.0736 | 257.0814 | 11.03 | +ve | [C15H12O4]+H, m/z 239.0707, m/z 215.0715, m/z 163.0399, m/z 137.0239, m/z 119.0498 |
| 14 | Ellagic acid | C14H6O8 | 302.0063 | 300.9986 | 11.03 | −ve | [C14H6O8]−H, m/z 283.9943, m/z 178.9969, m/z 151.0027, m/z 135.0080 |
| 15 | Quercetin-3-O-β-d-glucuronide | C21H18O13 | 478.0747 | 479.0826 | 11.81 | +ve | [C21H18O13]+H, m/z 303.0506, m/z 245.0452, m/z 147.0448 |
| 477.0677 | 11.77 | −ve | [C21H18O13]−H, m/z 301.0336, m/z 299.0180, m/z 243.0281, m/z 151.0025 | ||||
| 16 | Coumarin | C9H6O2 | 146.0368 | 147.0446 | 12.88 | +ve | [C9H6O2]+H, m/z 131.0499 |
| 17 | Kushenol O | C27H30O13 | 562.1686 | 563.1763 | 13.67 | +ve | [C27H30O13]+H, m/z 549.1600, m/z 387.1322, m/z 269.0813, m/z 237.0543, m/z 153.0719 |
| 561.1619 | 13.65 | −ve | [C27H30O13]−H, m/z 547.1428, m/z 401.0868, m/z 267.0648, m/z 252.0410, m/z 151.0391 | ||||
| 18 | Licurazide | C26H30O13 | 550.1686 | 551.1762 | 13.77 | +ve | [C26H30O13]+H, m/z 461.1421, m/z 419.1335, m/z 317.0667, m/z 257.0812, m/z 239.0705, m/z 137.0238 |
| 549.1616 | 13.74 | −ve | [C26H30O13]−H, m/z 417.1159, m/z 357.0962, m/z 255.0650, m/z 254.0566, m/z 135.0082 | ||||
| 19 | Liquiritin apioside | C26H30O13 | 550.1686 | 551.1757 | 14.07 | +ve | [C26H30O13]+H, m/z 453.1153, m/z 419.1333, m/z 389.1236, m/z 269.0813, m/z 257.0813, m/z 137.0238 |
| 549.1614 | 14.04 | −ve | [C26H30O13]−H, m/z 533.1630, m/z 399.1061, m/z 255.0651, m/z 165.0549, m/z 135.008 | ||||
| 20 | Liquiritin | C21H22O9 | 418.1264 | 419.1344 | 14.51 | +ve | [C21H22O9]+H, m/z 355.1184, m/z 257.0811, m/z 255.0655, m/z 147.0446 |
| 417.1191 | 14.47 | −ve | [C21H22O9]−H, m/z 343.1189, m/z 299.0544, m/z 255.0650, m/z 253.0490, m/z 163.0387, m/z 135.0079 | ||||
| 21 | N-feruloyltyramine | C18H19NO4 | 313.1314 | 314.1395 | 14.83 | +ve | [C18H19NO4]+H, m/z 177.0552, m/z 145.0289, m/z 121.0652 |
| 312.1240 | 14.80 | −ve | [C18H19NO4]−H, m/z 297.0988, m/z 178.0501, m/z 148.0520 | ||||
| 22 | Cinnamic acid | C9H8O2 | 148.0524 | 149.0603 | 15.71 | +ve | [C9H8O2]+H, m/z 131.0498 |
| 23 | 24-Hydroxy-licoricesaponin A3 | C48H72O22 | 1000.4515 | 999.4485 | 16.86 | −ve | [C48H72O22]−H, m/z 939.4566, m/z 819.3776, m/z 485.3237, m/z 373.1632, m/z 179.0701 |
| 24 | Licoricesaponin A3 | C48H72O21 | 984.4566 | 985.4633 | 17.71 | +ve | [C48H72O21]+H, m/z 866.3528, m/z 809.4295, m/z 615.3875, m/z 453.3357, m/z 435.3246, m/z 153.0184 |
| 983.4525 | 17.72 | −ve | [C48H72O21]−H, m/z 645.3610, m/z 469.3300, m/z 351.0545, m/z 193.0348 | ||||
| 25 | Glabrolide | C30H44O4 | 468.3240 | 469.3319 | 18.46 | +ve | [C30H44O4]+H, m/z 439.3570, m/z 405.3154, m/z 315.1961, m/z 233.1539, m/z 175.1485, m/z 149.1327 |
| 26 | Eugenol | C10H12O2 | 164.0837 | 164.0838 | 19.26 | +ve | [C10H12O2]-e, m/z 149.0603, m/z 131.0498, m/z 119.0497 |
| 27 | Piperanine | C17H21NO3 | 287.1521 | 288.1608 | 19.40 | +ve | [C17H21NO3]+H, m/z 256.1340, m/z 203.0709, m/z 171.0440, m/z 137.0604 |
| 28 | Licoricesaponin G2 | C42H62O17 | 838.3987 | 839.4069 | 19.88 | +ve | [C42H62O17]+H, m/z 582.2634, m/z 487.3414, m/z 469.3309, m/z 189.1641, m/z 175.1484 |
| 837.3944 | 19.89 | −ve | [C42H62O17]−H, m/z 793.3981, m/z 623.2339, m/z 431.2272, m/z 351.0551, m/z 193.0342 | ||||
| 29 | Glycyrrhizin | C42H62O16 | 822.4038 | 823.4115 | 20.71 | +ve | [C42H62O16]+H, m/z 700.4142, m/z 647.3781, m/z 453.3364, m/z 435.3262, m/z 272.1290, m/z, 189.1645 |
| 821.3994 | 20.69 | −ve | [C42H62O16]−H, m/z 759.3939, m/z 645.3619, m/z 499.3038, m/z 351.0555, m/z 193.0348 | ||||
| 30 | Piperyline | C16H17NO3 | 271.1208 | 272.1293 | 20.84 | +ve | [C16H17NO3]+H, m/z 244.1349, m/z 242.1165, m/z 201.0551, m/z 171.0447, m/z 135.0449, m/z 122.0360 |
| 31 | 3-O-(β-d-Glucuronopyranosyl-(1-2)-β-d-galactopyranosyl)glycyrrhetic acid | C42H64O15 | 808.4245 | 809.4319 | 21.41 | +ve | [C42H64O15]+H, m/z 633.3987, m/z 439.3571, m/z 437.3407, m/z 241.0879, m/z 175.1114 |
| 807.4197 | 21.42 | −ve | [C42H64O15]−H, m/z 745.4132, m/z 485.3251, m/z 303.2322, m/z 187.0961 | ||||
| 32 | Licoricesaponine K2 | C42H62O16 | 822.4038 | 823.4114 | 21.51 | +ve | [C42H62O16]+H, m/z 700.4185, m/z 647.3779, m/z 453.3364, m/z 435.3259, m/z 235.1698, m/z 189.1644 |
| 821.3991 | 21.52 | −ve | [C42H62O16]−H, m/z 807.4142, m/z 645.3607, m/z 485.3251, m/z 351.0550, m/z 193.0344 | ||||
| 33 | 6-Gingerol | C17H26O4 | 294.1831 | 317.1738 | 21.66 | +ve | [C17H26O4]+Na, m/z 259.1702, m/z 177.0917, m/z 162.0680, m/z 137.0605 |
| 34 | 4,5-Dihydropiperlonguminine | C16H21NO3 | 275.1521 | 276.1604 | 22.03 | +ve | [C16H21NO3]+H, m/z 246.1507, m/z 203.0712, m/z 135.0446, m/z 131.0494 |
| 35 | Piperlonguminine | C16H19NO3 | 273.1365 | 274.1448 | 22.29 | +ve | [C16H19NO3]+H, m/z 262.1438, m/z 201.0549, m/z 171.0446, m/z 135.0447, m/z 115.0992 |
| 36 | Licoricesaponine J2 | C42H64O16 | 824.4194 | 825.4265 | 22.53 | +ve | [C42H64O16]+H, m/z 613.3720, m/z 455.3516, m/z 409.3463, m/z 205.1061 |
| 823.4147 | 22.53 | −ve | [C42H64O16]−H, m/z 761.4095, m/z 597.2575, m/z 439.1797, m/z 351.0551, m/z 193.0346, m/z 175.0214 | ||||
| 37 | Feruperine | C17H21NO3 | 287.1521 | 288.1602 | 22.72 | +ve | [C17H21NO3]+H, m/z 270.1496, m/z 217.1090, m/z 203.0709, m/z 135.0447, m/z 124.0768 |
| 38 | Licoricesaponin C2 | C42H62O15 | 806.4089 | 829.3991 | 22.94 | +ve | [C42H62O15]+Na, m/z 560.3732, m/z 437.3411, m/z 396.2542, m/z 285.1852, m/z 173.0946 |
| 805.4042 | 22.95 | −ve | [C42H62O15]−H, m/z 743.3975, m/z 645.3662, m/z 501.3191, m/z 351.0552, m/z 167.0342 | ||||
| 39 | Piperine | C17H19NO3 | 285.1365 | 286.1449 | 23.13 | +ve | [C17H19NO3]+H, m/z 258.1495, m/z 201.0552, m/z 171.0447, m/z 135.0449, m/z 112.0763 |
| 40 | Shinpterocarpin | C20H18O4 | 322.1205 | 321.1135 | 23.28 | −ve | [C20H18O4]−H, m/z 306.0883, m/z 265.0490, m/z 237.0542, m/z 175.0758, m/z 145.0290 |
| 41 | Licoricesaponin B2 | C42H64O15 | 808.4245 | 831.4131 | 23.34 | +ve | [C42H64O15]+Na, m/z 731.3659, m/z 602.2705, m/z 485.3259, m/z 439.3567, m/z 279.1421, m/z 213.1123 |
| 807.4201 | 23.35 | −ve | [C42H64O15]−H, m/z 779.4222, m/z 695.3628, m/z 473.2729, m/z 351.0551, m/z 193.0343 | ||||
| 42 | Glabridin | C20H20O4 | 324.1362 | 325.1445 | 25.28 | +ve | [C20H20O4]+H, m/z 309.1130, m/z 270.0883, m/z 189.0916, m/z 173.0606, m/z 123.0447 |
| 323.1292 | 25.26 | −ve | [C20H20O4]−H, m/z 308.1037, m/z 268.0723, m/z 201.0915, m/z 135.0449 | ||||
| 43 | Piperettine | C19H21NO3 | 311.1521 | 312.1605 | 25.59 | +ve | [C19H21NO3]+H, m/z 294.1501, m/z 227.0709, m/z 197.0603, m/z 161.0602, m/z 138.0920 |
| 44 | Piperolein A | C19H25NO3 | 315.1834 | 316.1921 | 26.29 | +ve | [C19H25NO3]+H, m/z 231.1025, m/z 194.1547, m/z 135.0448, m/z 131.0497 |
| 45 | Dipiperamide E | C34H38N2O6 | 570.2730 | 571.2809 | 26.41 | +ve | [C34H38N2O6]+H, m/z 444.1771, m/z 286.1444, m/z 201.0520, m/z 173.0559 |
| 46 | Retrofractamide A | C20H25NO3 | 327.1834 | 328.1919 | 27.05 | +ve | [C20H25NO3]+H, m/z 227.1072, m/z 187.0758, m/z 161.0602, m/z 131.0498 |
| 47 | Glabrol | C25H28O4 | 392.1988 | 393.2070 | 27.31 | +ve | [C25H28O4]+H, m/z 337.1442, m/z 321.1129, m/z 281.0814, m/z 203.0708, m/z 149.0240, m/z 137.0604 |
| 391.1922 | 27.29 | −ve | [C25H28O4]−H, m/z 203.0707, m/z 187.1122, m/z 132.0577 | ||||
| 48 | 1-Methoxyphaseollidin | C21H22O5 | 354.1467 | 355.1551 | 27.58 | +ve | [C21H22O5]+H, m/z 265.0494, m/z 189.0912, m/z 153.0557 |
| 353.1397 | 27.55 | −ve | [C21H22O5]−H, m/z 295.0591, m/z 201.0911, m/z 150.0315 | ||||
| 49 | Piperolactam-C9:1(8E) | C20H27NO3 | 329.1991 | 330.2071 | 27.81 | +ve | [C20H27NO3]+H, m/z 259.1323, m/z 208.1702, m/z 135.0446, m/z 133.0650 |
| 50 | 1-Methoxyphaseollin | C21H20O5 | 352.1311 | 351.1239 | 27.86 | −ve | [C21H20O5]−H, m/z 321.1108, m/z 267.0644, m/z 201.0913, m/z 146.0356 |
| 51 | Dehydropipernonaline | C21H25NO3 | 339.1834 | 340.1915 | 28.34 | +ve | [C21H25NO3]+H, m/z 286.1445, m/z 227.1071, m/z 179.1310, m/z 161.0602, m/z 112.0761 |
| 52 | Pipernonaline | C21H27NO3 | 341.1991 | 342.2072 | 29.38 | +ve | [C21H27NO3]+H, m/z 314.2119, m/z 229.1227, m/z 161.0601, m/z 135.0447, m/z 112.0761 |
| 53 | 2α-Hydroxyursolic acid | C30H48O4 | 472.3553 | 471.3488 | 29.52 | −ve | [C30H48O4]−H, m/z 423.3237, m/z 393.3123, m/z 279.2320 |
| 54 | Licochalcone A | C21H22O4 | 338.1518 | 339.1600 | 29.82 | +ve | [C21H22O4]+H, m/z 276.0771, m/z 229.1227, m/z 189.0913, m/z 137.0602 |
| 337.1449 | 29.79 | −ve | [C21H22O4]−H, m/z 322.1187, m/z 267.0662, m/z 201.0910, m/z 175.0756, m/z 134.0369 | ||||
| 55 | Dipiperamide D | C36H40N2O6 | 596.2886 | 597.2961 | 30.18 | +ve | [C36H40N2O6]+H, m/z 512.2070, m/z 334.1427, m/z 286.1444, m/z 186.0655 |
| 56 | Piperolein B | C21H29NO3 | 343.2147 | 344.2230 | 30.81 | +ve | [C21H29NO3]+H, m/z 286.1447, m/z 222.1860, m/z 154.1234, m/z 135.0448 |
| 57 | Pipercide | C22H29NO3 | 355.2147 | 356.2231 | 31.16 | +ve | [C22H29NO3]+H, m/z 283.1334, m/z 255.1387, m/z 234.1858, m/z 135.0448, m/z 133.1014 |
| 58 | 10,11-Dihydropipercide | C22H31NO3 | 357.2304 | 358.2385 | 32.50 | +ve | [C22H31NO3]+H, m/z 285.1489, m/z 191.1066, m/z 135.0445 |
| 59 | Sophoranodichromane D | C25H28O5 | 408.1937 | 407.1865 | 32.73 | −ve | [C25H28O5]−H, m/z 350.1141, m/z 203.1064, m/z 203.0696, m/z 148.0522 |
| 60 | Piperundecalidine | C23H29NO3 | 367.2147 | 368.2232 | 33.25 | +ve | [C23H29NO3]+H, m/z 340.2281, m/z 255.1386, m/z 215.1071, m/z 135.0447, m/z 133.1011 |
| 61 | Shinflavanone | C25H26O4 | 390.1831 | 391.1912 | 36.31 | +ve | [C25H26O4]+H, m/z 375.1594, m/z 257.0773, m/z 215.1072, m/z 189.0914, m/z 147.0810 |
| 62 | Guineesine | C24H33NO3 | 383.2460 | 384.2543 | 36.61 | +ve | [C24H33NO3]+H, m/z 311.1648, m/z 283.1702, m/z 257.1535, m/z 175.0757, m/z 135.0447, m/z 131.0497 |
| 63 | Glycyrrhetic acid | C30H46O4 | 470.3396 | 471.3471 | 36.90 | +ve | [C30H46O4]+H, m/z 407.3320, m/z 364.3158, m/z 229.1937, m/z 175.1489, m/z 173.1333 |
| 469.3325 | 36.85 | −ve | [C30H46O4]−H, m/z 451.3185, m/z 407.3289 | ||||
| 64 | Ursolic acid | C30H48O3 | 456.3604 | 455.3538 | 38.72 | −ve | [C30H48O3]−H, m/z 389.3044, m/z 331.2605, m/z 125.0969 |
| 65 | Glycyrrhetol | C30H48O3 | 456.3604 | 455.3538 | 39.61 | −ve | [C30H48O3]−H, m/z 407.3301 |
| 66 | Liquidambronal | C30H46O2 | 438.3498 | 439.3578 | 39.68 | +ve | [C30H46O2]+H, m/z 408.3381, m/z 297.2555, m/z 255.2120, m/z 203.1800, m/z 191.1800, m/z 135.1173 |
| 67 | Betulonic acid | C30H46O3 | 454.3447 | 453.3387 | 42.87 | −ve | [C30H46O3]−H, m/z 301.2136, m/z 247.2058 |
| 68 | Oleanonic acid | C30H46O3 | 454.3447 | 455.3511 | 43.51 | +ve | [C30H46O3]+H, m/z 409.3453, m/z 343.2649, m/z 261.2222, m/z 203.1799, m/z 177.1643 |
| 453.3384 | 43.44 | −ve | [C30H46O3]−H, m/z 422.2805 | ||||
| 69 | Deoxyglabrolide | C30H46O3 | 454.3447 | 455.3522 | 49.70 | +ve | [C30H46O3]+H, m/z 437.3415, m/z 353.2489, m/z 321.2565, m/z 215.1799, m/z 189.1644, m/z 161.1330 |
| 453.3387 | 49.60 | −ve | [C30H46O3]−H, m/z 393.3134, m/z 317.2845, m/z 245.1536, m/z 177.0910, m/z 153.1281 | ||||
| 70 | Glypallidifloric acid | C30H46O3 | 454.3447 | 455.3521 | 50.49 | +ve | [C30H46O3]+H, m/z 437.3417, m/z 353.2487, m/z 297.2582, m/z 203.1800, m/z 161.1330, m/z 135.1175 |
| 453.3388 | 50.40 | −ve | [C30H46O3]−H, m/z 393.3133, m/z 167.1100 | ||||
| 71 | 5-Hydroxyeicosatetraenoic acid | C20H32O3 | 320.2351 | 319.2287 | 50.50 | −ve | [C20H32O3]−H, m/z 275.2378, m/z 273.2217, m/z 205.1217, m/z 153.1275 |
| 72 | Ginkgolic acid | C22H34O3 | 346.2508 | 347.2590 | 51.83 | +ve | [C22H34O3]+H, m/z 329.2486, m/z 233.1530, m/z 189.0919, m/z 161.0603, m/z 133.0294 |
| 345.2442 | 51.73 | −ve | [C22H34O3]−H, m/z 301.2531, m/z 299.2372, m/z 203.1433, m/z 175.1123, m/z 133.0651 | ||||
| 73 | N-Isobutyl-(2E,4E)-octadecadienamide | C22H41NO | 335.3188 | 336.3278 | 54.54 | +ve | [C22H41NO]+H, m/z 322.3121, m/z 280.2647, m/z 182.1551, m/z 154.1237, m/z 135.1176 |
| 74 | Pipnoohine | C24H43NO | 361.3345 | 362.3438 | 55.42 | +ve | [C24H43NO]+H, m/z 348.3279, m/z 306.2809, m/z 264.2334, m/z 191.1805, m/z 154.1238, m/z 135.1178 |
| Parameters | Acceptance Criteria | Results Obtained | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | Protocatechuic Acid | Methyl Gallate | Ellagic Acid | Coumarin | Cinnamic Acid | Glycyrrhizin | Eugenol | 6-Gingerol | Piperine | Glabridin | ||
| Specificity | No interference at retention time | In compliance | ||||||||||
| Linearity | Correlation coefficient (r2) NLT 0.99 | 0.9992 | 0.9991 | 0.9992 | 0.9992 | 0.9982 | 0.9995 | 0.9974 | 0.9972 | 0.9975 | 0.9974 | 0.9992 |
| Range (μg/g) | 20.0–2000 | 20.0–2000 | 6.6–2000 | 20.0–2000 | 6.6–2000 | 3.0–2000 | 20.0–2000 | 20.0–2000 | 20.0–2000 | 6.6–2000 | 6.6–2000 | |
| Precision | ||||||||||||
| Intraday | %RSD NMT 2 | 1.13 | 0.32 | 0.34 | 0.67 | 0.96 | 0.49 | 1.55 | 1.16 | 0.13 | 0.86 | 0.93 |
| Interday | %RSD NMT 2 | 1.08 | 0.44 | 1.36 | 1.01 | 1.52 | 0.17 | 0.47 | 1.72 | 0.39 | 1.75 | 0.68 |
| Mean average recovery (%) | 90–110% | 96.12 | 95.29 | 93.60 | 94.65 | 95.30 | 95.43 | 97.40 | 97.54 | 94.47 | 92.75 | 100.13 |
| Ruggedness | NMT 10 | 1.13 | 1.91 | 2.79 | 3.26 | 3.94 | 6.92 | 3.79 | 2.05 | 6.87 | 4.20 | 5.22 |
| Robustness | ||||||||||||
| Flow rate | %RSD NMT 20 | 2.66 | 9.56 | 15.63 | 6.41 | 5.26 | 6.86 | 7.80 | 2.13 | 4.65 | 2.70 | 7.48 |
| Column temperature | %RSD NMT 20 | 5.51 | 9.61 | 15.15 | 4.09 | 5.18 | 3.23 | 3.74 | 1.72 | 4.05 | 5.60 | 8.47 |
| Limit of Detection (LOD) | %RSD of area NMT 33 | 1.53 | 1.51 | 0.51 | 1.42 | 0.49 | 0.76 | 3.35 | 0.81 | 6.11 | 0.98 | 0.68 |
| LOD (μg/g) | 0.33 | 0.33 | 0.11 | 0.33 | 0.11 | 0.05 | 0.33 | 0.33 | 0.33 | 0.11 | 0.11 | |
| Limit of Quantification (LOQ) | %RSD of area NMT 10 | 0.60 | 0.93 | 1.10 | 1.48 | 0.99 | 1.64 | 1.02 | 0.52 | 0.38 | 1.28 | 0.48 |
| LOQ (μg/g) | 1.0 | 1.0 | 0.33 | 1.0 | 0.33 | 0.15 | 1.0 | 1.0 | 1.0 | 0.33 | 0.33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Verma, S.; Sharma, P.; Tomer, M.; Srivastava, J.; Varshney, A. Comprehensive and Rapid Quality Evaluation Method for the Ayurvedic Medicine Divya-Swasari-Vati Using Two Analytical Techniques: UPLC/QToF MS and HPLC–DAD. Pharmaceuticals 2021, 14, 297. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14040297
Balkrishna A, Verma S, Sharma P, Tomer M, Srivastava J, Varshney A. Comprehensive and Rapid Quality Evaluation Method for the Ayurvedic Medicine Divya-Swasari-Vati Using Two Analytical Techniques: UPLC/QToF MS and HPLC–DAD. Pharmaceuticals. 2021; 14(4):297. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14040297
Chicago/Turabian StyleBalkrishna, Acharya, Sudeep Verma, Priyanka Sharma, Meenu Tomer, Jyotish Srivastava, and Anurag Varshney. 2021. "Comprehensive and Rapid Quality Evaluation Method for the Ayurvedic Medicine Divya-Swasari-Vati Using Two Analytical Techniques: UPLC/QToF MS and HPLC–DAD" Pharmaceuticals 14, no. 4: 297. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14040297






