Periodontal Wound Healing and Tissue Regeneration: A Narrative Review
Abstract
:1. Introduction
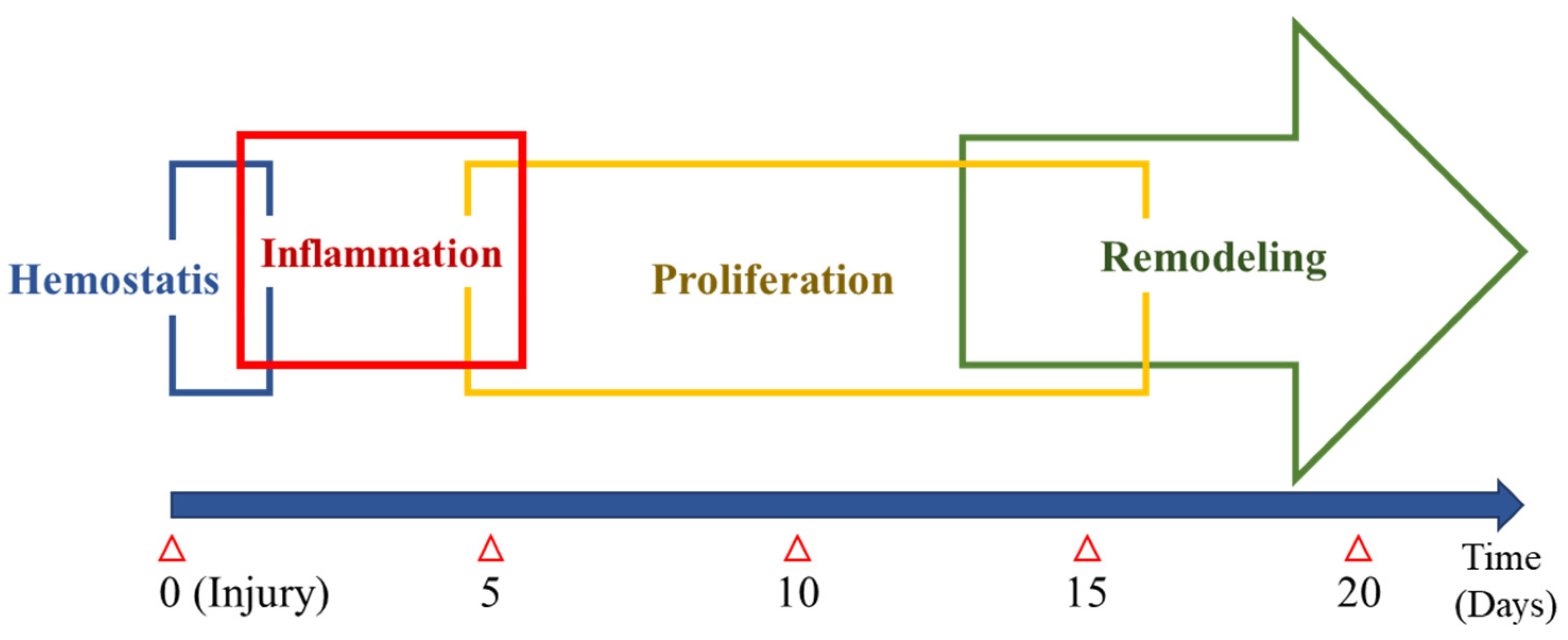
2. Normal Wound Healing
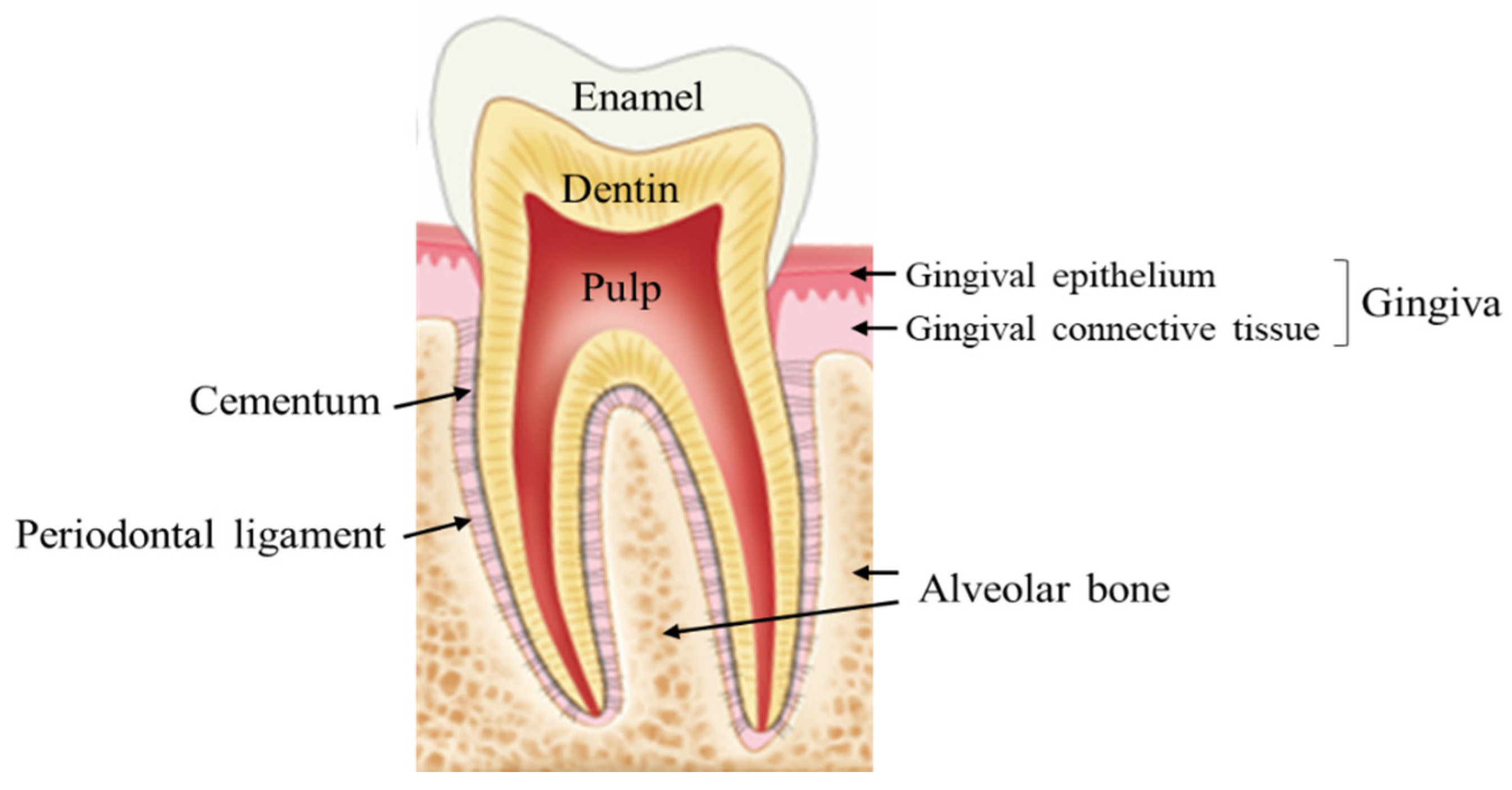
3. Distinct Characteristics of the Oral Wound
3.1. Attenuated Inflammatory Reaction
3.2. Differential Angiogenesis Pattern
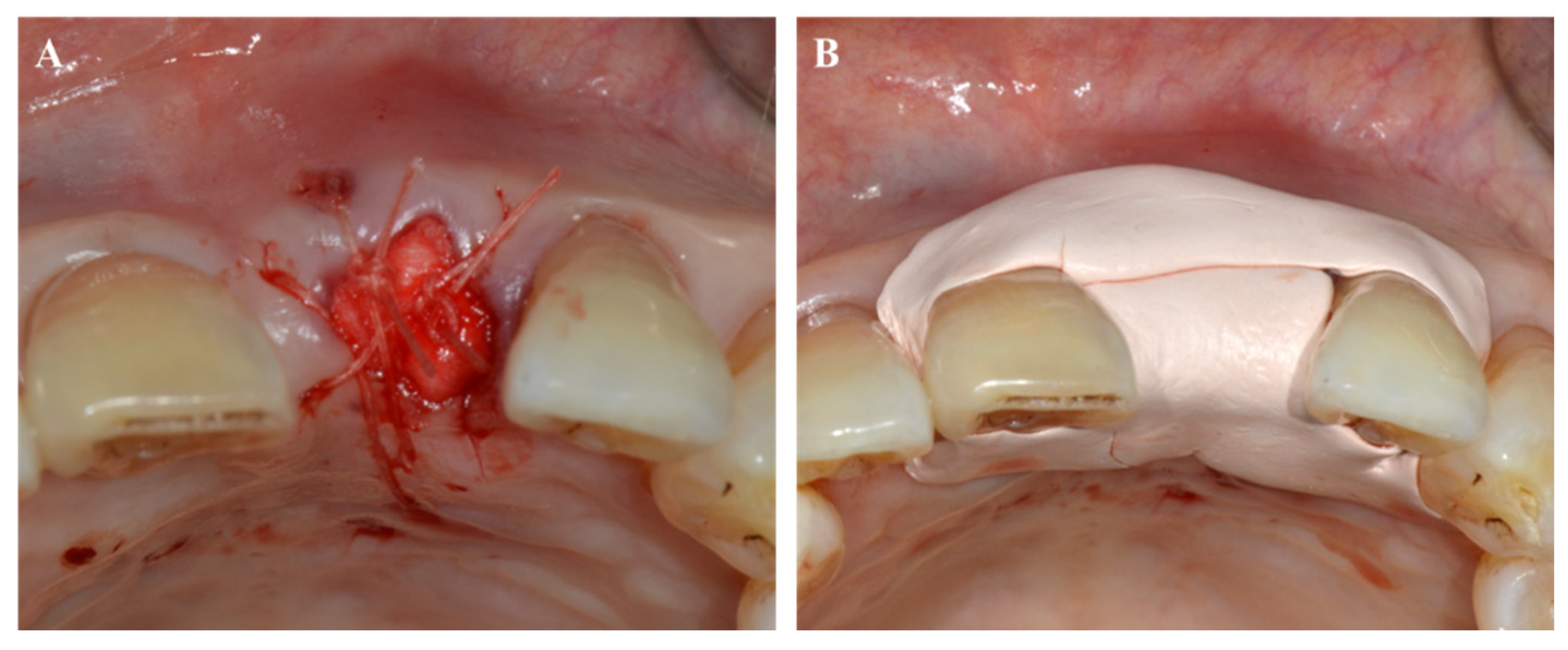
4. Periodontal Treatment and Wound Healing
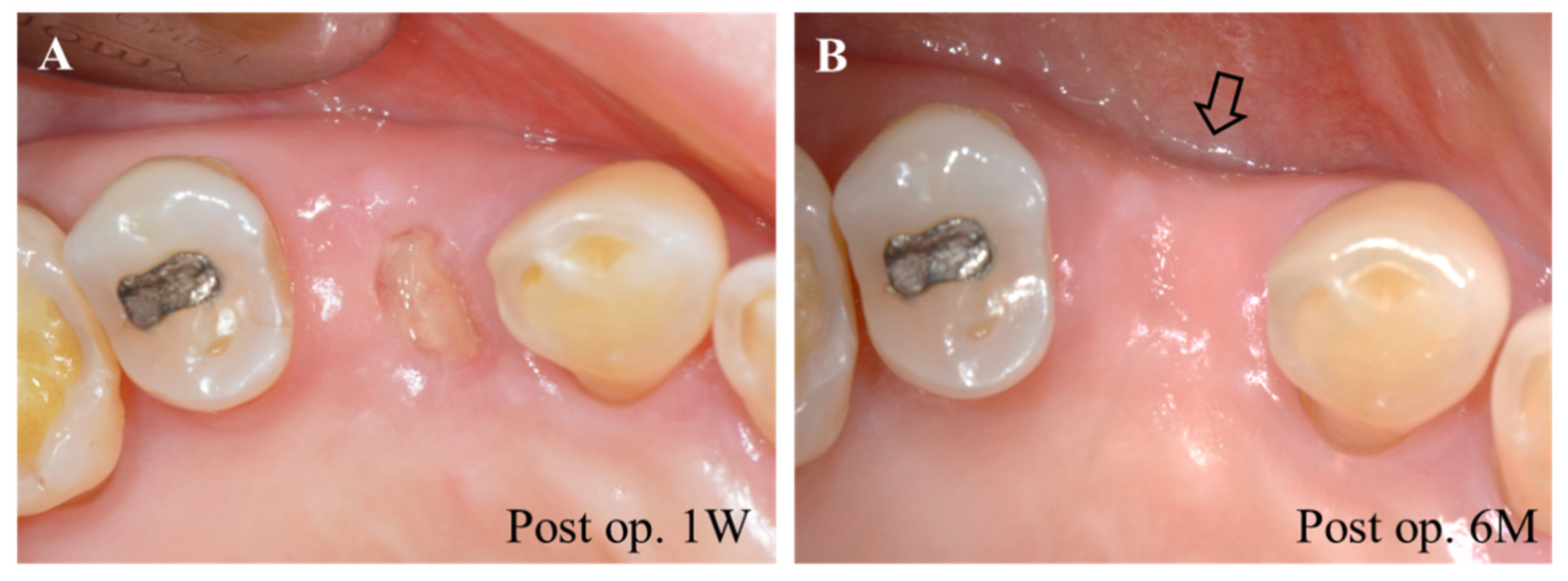
4.1. Tooth Extraction
4.2. Resective Periodontal Surgery
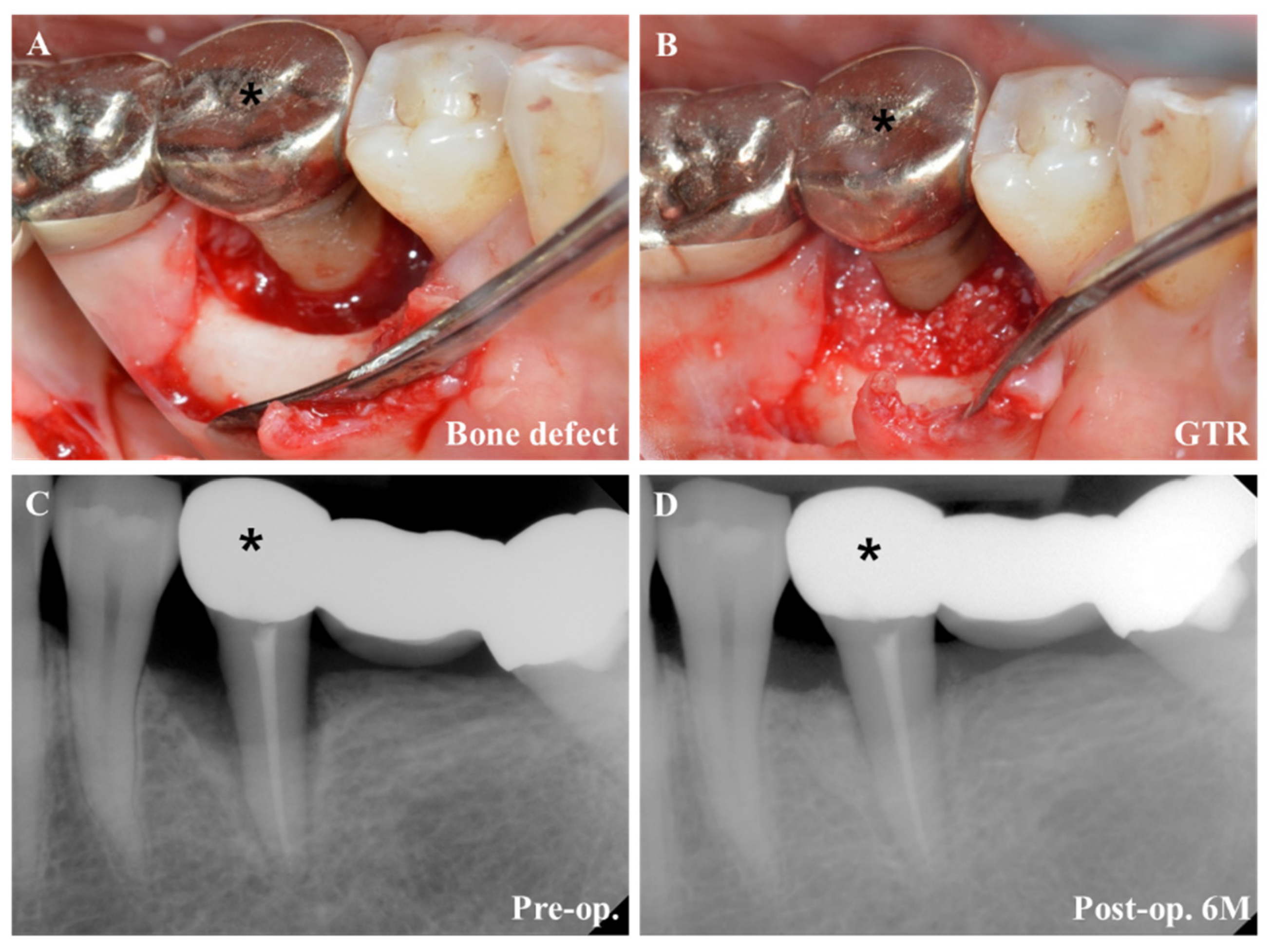
4.3. Regenerative Periodontal Surgery
5. Complications after Periodontal Treatment
5.1. Postoperative Infection
5.2. Bleeding
5.3. Swelling
5.4. Scar Formation
6. Factors Affecting Periodontal Wound Healing
6.1. Vascularization, Flap Design, and Incision
6.2. Aging (Senescence)
6.3. Diabetes Mellitus (DM)
6.4. Smoking
7. Therapeutics for Periodontal Wound Healing
7.1. Biopharmaceutical Approaches
7.1.1. Enamel Matrix Derivative (EMD)
7.1.2. Collagen
7.1.3. Blood-Derived Products
- 1.
- PRP
- 2.
- PRGF
- 3.
- Fibrin Sealant
7.2. Periodontal Dressing Materials
7.3. Devices to Improve Wound Healing
7.3.1. Light Amplification by Stimulated Emission of Radiation (Laser)
7.3.2. Hyperbaric Oxygen
8. Perspective and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Harvey, J.D. Periodontal Microbiology. Dent. Clin. N. Am. 2017, 61, 253–269. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Dietrich, T.; Ower, P.; Tank, M.; West, N.X.; Walter, C.; Needleman, I.; Hughes, F.J.; Wadia, R.; Milward, M.R.; Hodge, P.J.; et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions—Implementation in clinical practice. Br. Dent. J. 2019, 226, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.D.; Kim, W.J.; Ryoo, H.M.; Kim, H.G.; Kim, K.H.; Ku, Y.; Seol, Y.J. Current advances of epigenetics in periodontology from ENCODE project: A review and future perspectives. Clin. Epigenet. 2021, 13, 92. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjo, U.M. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000 2006, 41, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, G.; Creber, K.; Hamilton, D.W. Wound healing and fibrosis: A contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 2020, 318, C1065–C1077. [Google Scholar] [CrossRef]
- Politis, C.; Schoenaers, J.; Jacobs, R.; Agbaje, J.O. Wound Healing Problems in the Mouth. Front. Physiol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias-Bartolome, R.; Uchiyama, A.; Molinolo, A.A.; Abusleme, L.; Brooks, S.R.; Callejas-Valera, J.L.; Edwards, D.; Doci, C.; Asselin-Labat, M.L.; Onaitis, M.W.; et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Katsani, K.R.; Sakellari, D. Saliva proteomics updates in biomedicine. J. Biol. Res. 2019, 26, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahangar, P.; Mills, S.J.; Smith, L.E.; Gronthos, S.; Cowin, A.J. Human gingival fibroblast secretome accelerates wound healing through anti-inflammatory and pro-angiogenic mechanisms. NPJ Regen Med. 2020, 5, 24. [Google Scholar] [CrossRef]
- Chen, L.; Arbieva, Z.H.; Guo, S.; Marucha, P.T.; Mustoe, T.A.; DiPietro, L.A. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genom. 2010, 11, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, G.; Neumann, P.A.; Sumagin, R.; Denning, T.L.; Nusrat, A. Wound repair: Role of immune-epithelial interactions. Mucosal Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [Green Version]
- desJardins-Park, H.E.; Mascharak, S.; Chinta, M.S.; Wan, D.C.; Longaker, M.T. The Spectrum of Scarring in Craniofacial Wound Repair. Front. Physiol. 2019, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Turabelidze, A.; Guo, S.J.; Chung, A.Y.; Chen, L.; Dai, Y.; Marucha, P.T.; DiPietro, L.A. Intrinsic Differences between Oral and Skin Keratinocytes. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Glim, J.E.; Beelen, R.H.; Niessen, F.B.; Everts, V.; Ulrich, M.M. The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Arch. Oral Biol. 2015, 60, 272–281. [Google Scholar] [CrossRef]
- Boink, M.A.; van den Broek, L.J.; Roffel, S.; Nazmi, K.; Bolscher, J.G.; Gefen, A.; Veerman, E.C.; Gibbs, S. Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair Regen. 2016, 24, 100–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpaderska, A.M.; Zuckerman, J.D.; DiPietro, L.A. Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 2003, 82, 621–626. [Google Scholar] [CrossRef]
- Chen, L.; Gajendrareddy, P.K.; DiPietro, L.A. Differential expression of HIF-1alpha in skin and mucosal wounds. J. Dent. Res. 2012, 91, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Pippi, R. Post-Surgical Clinical Monitoring of Soft Tissue Wound Healing in Periodontal and Implant Surgery. Int. J. Med. Sci. 2017, 14, 721–728. [Google Scholar] [CrossRef] [Green Version]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and Cellular Aspects of Socket Healing in the Absence and Presence of Graft Materials and Autologous Platelet Concentrates: A Focused Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Kim, J.H.; Susin, C.; Min, J.H.; Suh, H.Y.; Sang, E.J.; Ku, Y.; Wikesjo, U.M.; Koo, K.T. Extraction sockets: Erratic healing impeding factors. J. Clin. Periodontol. 2014, 41, 80–85. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Arunachalam, L.T.; Sudhakar, U. Minimally invasive surgery in periodontics—A review. IP Int. J. Periodontol. Implantol. 2019, 4, 130–137. [Google Scholar]
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Smitha Annie Jacob, A.D. Guided tissue regeneration: A review. J. Dent. Health Oral Disord. Ther. 2017, 6, 67–73. [Google Scholar]
- Suchetha, A.E.T.; Darshan, B.M.; Apoorva, S.M.; Divya, B. Post-operative complications after periodontal surgery. Int. J. Appl. Dent. Sci. 2018, 4, 152–156. [Google Scholar]
- Ria, B.; Wates, E.; Ria, S. A review of haemostasis following minor oral surgery procedures. J. Dent. Health Oral Disord. Ther. 2017, 7, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Jaisika Rajpal, A.A.; Ruchika, P.; Madhav Mukund, G. Preventing postoperative swelling after periodontal surgery. J. Oral. Res. Rev. 2015, 7, 31–34. [Google Scholar] [CrossRef]
- Chappi, D.M.; Suresh, K.V.; Patil, M.R.; Desai, R.; Tauro, D.P.; Bharani, K.N.S.S.; Parkar, M.I.; Babaji, H.V. Comparison of clinical efficacy of methylprednisolone and serratiopeptidase for reduction of postoperative sequelae after lower third molar surgery. J. Clin. Exp. Dent. 2015, 7, e197–e202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Krishna, S.; Khandeparker, R.V. A comprehensive management protocol to treat cleft maxillary hypoplasia. J. Craniomaxillofac. Surg. 2018, 46, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, A.; Feigl, G.; Sculean, A.; Grimm, A.; Palkovics, D.; Molnar, B.; Windisch, P. Vascular survey of the maxillary vestibule and gingiva-clinical impact on incision and flap design in periodontal and implant surgeries. Clin. Oral Investig. 2021, 25, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, A.; Pilsl, U.; Molnar, B.; Feigl, G. Detection of Vascular Pathways of Oral Mucosa Influencing Soft- and Hard Tissue Surgeries by Latex Milk Injection. J. Vis. Exp. Jove 2020. [Google Scholar] [CrossRef] [PubMed]
- Zuhr, O.; Rebele, S.F.; Cheung, S.L.; Hurzeler, M.B.; Research Group on Oral Soft Tissue, B.; Wound, H. Surgery without papilla incision: Tunneling flap procedures in plastic periodontal and implant surgery. Periodontol 2000 2018, 77, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Kleinheinz, J.; Buchter, A.; Kruse-Losler, B.; Weingart, D.; Joos, U. Incision design in implant dentistry based on vascularization of the mucosa. Clin. Oral Implant. Res. 2005, 16, 518–523. [Google Scholar] [CrossRef]
- Sheikh, R.; Memarzadeh, K.; Torbrand, C.; Blohme, J.; Malmsjo, M. Hypoperfusion in response to epinephrine in local anaesthetics: Investigation of dependence on epinephrine concentration, spread of hypoperfusion and time to maximal cutaneous vasoconstriction. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 322–329. [Google Scholar] [CrossRef]
- Mikecs, B.; Vag, J.; Gerber, G.; Molnar, B.; Feigl, G.; Shahbazi, A. Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health 2021, 21, 160. [Google Scholar] [CrossRef]
- Sousounis, K.; Baddour, J.A.; Tsonis, P.A. Aging and regeneration in vertebrates. Curr. Top. Dev. Biol. 2014, 108, 217–246. [Google Scholar] [CrossRef]
- Levi, N.; Papismadov, N.; Solomonov, I.; Sagi, I.; Krizhanovsky, V. The ECM path of senescence in aging: Components and modifiers. FEBS J. 2020, 287, 2636–2646. [Google Scholar] [CrossRef]
- Smith, P.C.; Caceres, M.; Martinez, C.; Oyarzun, A.; Martinez, J. Gingival wound healing: An essential response disturbed by aging? J. Dent. Res. 2015, 94, 395–402. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Catrina, S.B.; Zheng, X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 179–185. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhao, F.; Zhang, Y.; Wang, Z. HIF1alpha overexpression enhances diabetic wound closure in high glucose and low oxygen conditions by promoting adipose-derived stem cell paracrine function and survival. Stem Cell. Res. Ther. 2020, 11, 148. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P. The impact of smoking on wound healing: The role of the nurse. Br. J. Nurs. 2018, 27, S10–S14. [Google Scholar] [CrossRef]
- Naji, A.; Edman, K.; Holmlund, A. Influence of smoking on periodontal healing one year after active treatment. J. Clin. Periodontol. 2020, 47, 343–350. [Google Scholar] [CrossRef]
- Ng, T.K.; Huang, L.; Cao, D.; Yip, Y.W.; Tsang, W.M.; Yam, G.H.; Pang, C.P.; Cheung, H.S. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci. Rep. 2015, 5, 7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.D.; Kim, P.J.; Kim, H.G.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Ryoo, H.M. Transcriptomics and methylomics in chronic periodontitis with tobacco use: A pilot study. Clin. Epigenet. 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.D.; Kim, P.J.; Kim, H.G.; Seol, Y.J.; Lee, Y.M.; Ryoo, H.M.; Ku, Y. Transcriptome and methylome analysis of periodontitis and peri-implantitis with tobacco use. Gene 2020, 727, 144258. [Google Scholar] [CrossRef]
- Kubota, M.; Yanagita, M.; Mori, K.; Hasegawa, S.; Yamashita, M.; Yamada, S.; Kitamura, M.; Murakami, S. The Effects of Cigarette Smoke Condensate and Nicotine on Periodontal Tissue in a Periodontitis Model Mouse. PLoS ONE 2016, 11, e0155594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Deveci, B.; Ayna, B.; Tacir, I.H.; Deveci, E.; Tuncer, M.C.; Pala, A. Effects of nicotine administration in rats on MMP2 and VEGF levels in periodontal membrane. Folia Morphol. 2018, 77, 471–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozumi, T.; Kubota, T.; Sato, T.; Okuda, K.; Yoshie, H. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J. Clin. Periodontol. 2004, 31, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Kokubu, E.; Kita, D.; Ota, K.; Yoshikawa, K.; Ishihara, K.; Saito, A. Role of mitogen-activated protein kinase pathways in migration of gingival epithelial cells in response to stimulation by cigarette smoke condensate and infection by Porphyromonas gingivalis. J. Periodontal Res. 2016, 51, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.S.; Ismail, A. Nicotine has deleterious effects on wound healing through increased vasoconstriction. BMJ 2016, 353, i2709. [Google Scholar] [CrossRef]
- Villa, O.; Wohlfahrt, J.C.; Mdla, I.; Petzold, C.; Reseland, J.E.; Snead, M.L.; Lyngstadaas, S.P. Proline-Rich Peptide Mimics Effects of Enamel Matrix Derivative on Rat Oral Mucosa Incisional Wound Healing. J. Periodontol. 2015, 86, 1386–1395. [Google Scholar] [CrossRef]
- Seshima, F.; Aoki, H.; Takeuchi, T.; Suzuki, E.; Irokawa, D.; Makino-Oi, A.; Sugito, H.; Tomita, S.; Saito, A. Periodontal regenerative therapy with enamel matrix derivative in the treatment of intrabony defects: A prospective 2-year study. BMC Res. Notes 2017, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, G.F.; de Araujo, V.C.; Nery, J.C.; Peruzzo, D.C.; Soares, A.B. Microvessel Density Evaluation of the Effect of Enamel Matrix Derivative on Soft Tissue After Implant Placement: A Preliminary Study. Int. J. Periodontics Restor. Dent. 2015, 35, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Villa, O.; Wohlfahrt, J.C.; Koldsland, O.C.; Brookes, S.J.; Lyngstadaas, S.P.; Aass, A.M.; Reseland, J.E. EMD in periodontal regenerative surgery modulates cytokine profiles: A randomised controlled clinical trial. Sci. Rep. 2016, 6, 23060. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Mizutani, K.; Matsuura, T.; Kido, D.; Mikami, R.; Noda, M.; Buranasin, P.; Sasaki, Y.; Izumi, Y. Periodontal regenerative effect of enamel matrix derivative in diabetes. PLoS ONE 2018, 13, e0207201. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shan, T.; Ma, Y.X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, L.; Kurtzman, G.M.; Shukla, S. Regeneration in Periodontics: Collagen-A Review of Its Properties and Applications in Dentistry. Compend. Contin. Educ. Dent. 2015, 36, 358–363. [Google Scholar]
- Abdelaziz, M.; Shaaban, R.; Abdelhalim, S.; Sadaka, M. Effect of CollagPlug® on the healing of extraction sockets in patients under oral anticoagulant therapy. Alex. Dent. J. 2015, 40, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, M.; Balaji, M.; Krishnaraj, R.; Narayanan, V.; Thangavelu, A. Assessment of Regeneration of Bone in the Extracted Third Molar Sockets Augmented Using Xenograft (CollaPlug(TN) Zimmer) in Comparison with the Normal Healing on the Contralateral Side. J. Pharm. Bioallied Sci. 2017, 9, S180–S186. [Google Scholar] [CrossRef] [PubMed]
- Resnik, R.R. Intraoperative Complications: Bleeding; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cho, Y.D.; Seol, Y.J.; Lee, Y.M.; Heo, S.J.; Ku, Y. Immediate Implant Placement at a Periapical Lesion Site: A Case Series. J. Oral Implantol. 2018, 44, 281–286. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yun, P.Y.; Lee, H.J.; Ahn, J.Y.; Kim, S.G. Ridge preservation of the molar extraction socket using collagen sponge and xenogeneic bone grafts. Implant. Dent. 2011, 20, 267–272. [Google Scholar] [CrossRef]
- Chang, L.C.; Cheng, Y.M. The Effect of Different Socket Types on Implant Therapy While Using Flapless Ridge Preservation. Appl. Sci. Basel 2021, 11, 970. [Google Scholar] [CrossRef]
- Achneck, H.E.; Sileshi, B.; Jamiolkowski, R.M.; Albala, D.M.; Shapiro, M.L.; Lawson, J.H. A Comprehensive Review of Topical Hemostatic Agents Efficacy and Recommendations for Use. Ann. Surg. 2010, 251, 217–228. [Google Scholar] [CrossRef]
- Tomizawa, Y. Clinical benefits and risk analysis of topical hemostats: A review. J. Artif. Organs 2005, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wang, L.N.; Zhou, Z.Y.; Lai, H.J.; Xu, P.; Liao, L.; Wei, J.C. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Irokawa, D.; Takeuchi, T.; Noda, K.; Goto, H.; Egawa, M.; Tomita, S.; Sugito, H.; Nikaido, M.; Saito, A. Clinical outcome of periodontal regenerative therapy using collagen membrane and deproteinized bovine bone mineral: A 2.5-year follow-up study. BMC Res. Notes 2017, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, B.; Ruan, R.; Landao-Bassonga, E.; Gillman, N.; Wang, T.; Gao, J.; Ruan, Y.; Xu, Y.; Lee, C.; Goonewardene, M.; et al. Collagen Membrane for Guided Bone Regeneration in Dental and Orthopedic Applications. Tissue Eng. Part A 2021, 27, 372–381. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materialsl 2020, 13, 786. [Google Scholar] [CrossRef] [Green Version]
- Bubalo, M.; Lazic, Z.; Tatic, Z.; Milovic, R.; Magic, M. The use of collagen membranes in guided tissue regeneration. Vojnosanit. Pregl. 2017, 74, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.J.; Meraw, S.J.; Lee, E.J.; Giannobile, W.V.; Wang, H.L. Comparative analysis of collagen membranes for the treatment of implant dehiscence defects. Clin. Oral Implant. Res. 2003, 14, 80–90. [Google Scholar] [CrossRef]
- Silva, E.C.; Omonte, S.V.; Martins, A.G.; de Castro, H.H.; Gomes, H.E.; Zenobio, E.G.; de Oliveira, P.A.; Horta, M.C.; Souza, P.E. Hyaluronic acid on collagen membranes: An experimental study in rats. Arch. Oral Biol. 2017, 73, 214–222. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubery, Y.; Nir, E.; Goldlust, A. Ossification of a collagen membrane cross-linked by sugar: A human case series. J. Periodontol. 2008, 79, 1101–1107. [Google Scholar] [CrossRef]
- Almazrooa, S.A.; Noonan, V.; Woo, S.B. Resorbable collagen membranes: Histopathologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.; Kumar, T.S.; Arun, K.V.; Arun, R.; Karthik, S.J. Clinical and histological evaluation of two dressing materials in the healing of palatal wounds. J. Indian Soc. Periodontol. 2010, 14, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kumar, A.; Puri, K.; Bansal, M.; Khatri, M. Application of platelet-rich fibrin membrane and collagen dressing as palatal bandage for wound healing: A randomized clinical control trial. Indian J. Dent. Res. 2019, 30, 881–888. [Google Scholar] [CrossRef]
- Moustafa, H.; Omar, S.; Osman, S.; Kawana, K. EFFECT OF COLLATAPE® COLLAGEN WOUND DRESSING ALONE AND COMBINED WITH INGENIOS® SYNTHETIC BONE GRAFT ON THE SOCKET HEALING IN RABBITS. Alex. Dent. J. 2015, 40, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.P.; Jaishangar, N.; Devy, S.; Narayanan, A.; Cherian, D.; Madhavan, S.S. Platelet-Rich Plasma and Platelet-Rich Fibrin in Periodontal Regeneration: A Review. J. Pharm. Bioallied Sci. 2019, 11, S126–S130. [Google Scholar] [CrossRef]
- Jalaluddin, M.; Mahesh, J.; Mahesh, R.; Jayanti, I.; Faizuddin, M.; Kripal, K.; Nazeer, N. Effectiveness of Platelet Rich Plasma and Bone Graft in the Treatment of Intrabony Defects: A Clinico-radiographic Study. Open Dent. J. 2018, 12, 133–154. [Google Scholar] [CrossRef]
- Greenwell, M.A.E.a.H. Theoretical and Clinical Considerations for Autologous Blood Preparations: Platelet-Rich Plasma, Fibrin Sealants, and Plasma-Rich Growth Factors. Clin. Adv. Periodontics 2011, 1, 142–153. [Google Scholar]
- Jovani-Sancho, M.D.; Sheth, C.C.; Marques-Mateo, M.; Puche-Torres, M. Platelet-Rich Plasma: A Study of the Variables that May Influence Its Effect on Bone Regeneration. Clin. Implant. Dent. Relat. Res. 2016, 18, 1051–1064. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review-The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Purkayastha, A.; Mohanty, R.; Nayak, R.; Satpathy, A.; Das, A.C.; Kumar, M.; Mohanty, G.; Panda, S.; Fabbro, M.D. Plasma rich in growth factors (PRGF) in non-surgical periodontal therapy: A randomized clinical trial. Braz. Oral Res. 2020, 34, e034. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makogonenko, E.; Tsurupa, G.; Ingham, K.; Medved, L. Interaction of fibrin(ogen) with fibronectin: Further characterization and localization of the fibronectin-binding site. Biochemistry 2002, 41, 7907–7913. [Google Scholar] [CrossRef]
- Shaju Jacob, S.N. Fibrin Sealant: A Review of Its Applications i Periodontal Surgery. Int. J. Exp. Dent. Sci. 2015, 4, 40–46. [Google Scholar] [CrossRef]
- Daigo, Y.; Daigo, E.; Fukuoka, H.; Fukuoka, N.; Ishikawa, M.; Takahashi, K. Wound Healing and Cell Dynamics Including Mesenchymal and Dental Pulp Stem Cells Induced by Photobiomodulation Therapy: An Example of Socket-Preserving Effects after Tooth Extraction in Rats and a Literature Review. Int. J. Mol. Sci. 2020, 21, 6850. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Dalvi, S.; Benedicenti, S.; Hanna, R. Effectiveness of Photobiomodulation as an Adjunct to Nonsurgical Periodontal Therapy in the Management of Periodontitis- A Systematic Review of in vivo Human Studies. Photochem. Photobiol. 2021, 97, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in Periodontology and Implant Dentistry: Part 1. Photobiomodul. Photomed. Laser Surg. 2019, 37, 739–765. [Google Scholar] [CrossRef] [PubMed]
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of High-Intensity Laser Therapy Combined with Photobiomodulation Therapy for Socket Preservation After Tooth Extraction. Photobiomodul. Photomed. Laser Surg. 2020, 38, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Suter, V.G.A.; Sjolund, S.; Bornstein, M.M. Effect of laser on pain relief and wound healing of recurrent aphthous stomatitis: A systematic review. Lasers Med. Sci. 2017, 32, 953–963. [Google Scholar] [CrossRef]
- Cayan, T.; Hasanoglu Erbasar, G.N.; Akca, G.; Kahraman, S. Comparative Evaluation of Diode Laser and Scalpel Surgery in the Treatment of Inflammatory Fibrous Hyperplasia: A Split-Mouth Study. Photobiomodul. Photomed. Laser Surg. 2019, 37, 91–98. [Google Scholar] [CrossRef]
- Akpinar, A.; Toker, H.; Lektemur Alpan, A.; Calisir, M. Postoperative discomfort after Nd:YAG laser and conventional frenectomy: Comparison of both genders. Aust. Dent. J. 2016, 61, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, O.; Seydaoglu, G.; Haytac, C.M. Diode laser for harvesting de-epithelialized palatal graft in the treatment of gingival recession defects: A randomized clinical trial. J. Clin. Periodontol. 2016, 43, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Paknejad, M.; Jamali, R.; Nokhbatolfoghahaei, H.; Fekrazad, R.; Moslemi, N. Effect of laser photobiomodulation on wound healing and postoperative pain following free gingival graft: A split-mouth triple-blind randomized controlled clinical trial. J. Photochem. Photobiol. B 2017, 172, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, C.; Takedachi, M.; Kawasaki, K.; Shimomura, J.; Murata, M.; Hirai, A.; Kawakami, K.; Sawada, K.; Iwayama, T.; Murakami, S. Hypoxia stimulates collagen hydroxylation in gingival fibroblasts and periodontal ligament cells. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Taylor, C.T.; Doherty, G.; Fallon, P.G.; Cummins, E.P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Investig. 2016, 126, 3716–3724. [Google Scholar] [CrossRef] [Green Version]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019, 109, 440–447. [Google Scholar] [CrossRef]
- Dryden, M.; Cooke, J.; Salib, R.; Holding, R.; Pender, S.L.F.; Brooks, J. Hot topics in reactive oxygen therapy: Antimicrobial and immunological mechanisms, safety and clinical applications. J. Glob. Antimicrob. Resist. 2017, 8, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandawa, G.; Mustaqimah, D.N.; Sidik, S.; Saraswati, H.; Putri, F.A.; Auerkari, E.I. Efficacy of Hyperbaric Oxygen Therapy as an Adjunctive Therapy of Chronic Periodontitis. J. Int. Dent. Med. Res. 2017, 10, 72–75. [Google Scholar]
- Giacon, T.A.; Giancola, F.; Paganini, M.; Tiengo, C.; Camporesi, E.M.; Bosco, G. Hyperbaric Oxygen Therapy and A-PRF Pre-Treated Implants in Severe Periodontitis: A Case Report. Int. J. Environ. Res. Public Health 2021, 18, 413. [Google Scholar] [CrossRef]
| Oral Wound | Skin Wound | |
|---|---|---|
| Re-epithelialization | Re-epithelialization in oral wound is faster than skin wound | |
| Re-epithelialization (24 h) | 100% | 40% |
| Inflammation | Inflammatory reaction is reduced and resolution is faster in oral wound than skin | |
| Inflammatory cells (Neutrophils, T cells, Macrophages) | ↓ | ↑ |
| Cytokines (IL-1β, IL-6, IL-α, TNF-α) | ↓ | ↑ |
| Angiogenesis | Angiogenic response is decreased in oral wound | |
| Vessel density | ↓ | ↑ |
| VEGF | ↓ | ↑ |
| ECM | MMT/TIMP ratio is decreased in oral wound | |
| Matrix metalloproteinases (MMP) | ↓ | ↑ |
| Tissue inhibitor of metalloproteinase (TIMP) | ↑ | ↑ |
| SCAR | Reduced scar formation is observed in oral wound | |
| TGF-β1/β3 | ↓ | ↑ |
| Types | Application | Commercial Product (Manufacturer) | Reference |
|---|---|---|---|
| Sponge |
| CollaPlug (Integra LifeSciences Corp.) | [65,66] |
| OraPlug (Salvin) | [67] | ||
| Teruplug (Olympus Terumo Biomaterials) | [68,69,70] | ||
| Avitene Ultrafoam Collagen Sponge (Davol, Inc.) | [71,72] | ||
| Membrane |
| Bio-Gide (Geistlich) | [73,74,75,76,77,78] |
| BioMend/OsseoGuard (Zimmer Biomet Inc.) | [73,78,79] | ||
| Ossix (Datum Dental Ltd.) | [80,81] | ||
| Periogen (Collagen Corporation) | [73,82] | ||
| CollaCote/CollaTape (Integra LifeSciences Corp.) | [83,84,85] |
| Application | Type | Method | Effect | Ref. |
|---|---|---|---|---|
| Extraction socket | Combined HILT and PBMT | HILT (27 J) was performed immediately after tooth extraction to enhance blood coagulation, followed by PBMT (0.7 J) 1 day later to enhance healing | Combined HILT and PBMT following tooth extraction hastened wound healing and preserved alveolar crest height, suggesting a role in socket preservation | [99] |
| Recurrent aphthous stomatitis (RAS) | CO2 laser, Nd:YAG laser and diode laser | Laser treatment included Nd:YAG laser ablation, CO2 laser applied through a transparent gel (non-ablative) and diode laser in a low-level laser treatment (LLLT) mode | The use of lasers (CO2 laser, Nd:YAG laser and diode laser) to relieve symptoms and promote healing of RAS | [100] |
| Inflammatory fibrous hyperplasia | Diode laser systems | Randomized, split-mouth clinical trial; comparative evaluation of diode laser and scalpel surgery | Bleeding and bacterial count was low in the laser group | [101] |
| Frenectomy | Nd:YAG laser treatment | Randomized clinical trial on postoperative discomfort after Nd:YAG laser and conventional frenectomy | Nd:YAG laser treatment used for frenectomies provides better postoperative comfort (pain, chewing, talking) | [102] |
| Harvesting de-epithelialized palatal graft | Diode laser systems | Randomized clinical trial: comparative evaluation of diode laser and scalpel surgery | Laser technique decreased post-operative morbidity | [103] |
| Free gingival graft | PBMT | A split-mouth triple-blind randomized controlled clinical trial | PBMT accelerated the rate of epithelialization at the donor site | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14050456
Cho Y-D, Kim K-H, Lee Y-M, Ku Y, Seol Y-J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals. 2021; 14(5):456. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14050456
Chicago/Turabian StyleCho, Young-Dan, Kyoung-Hwa Kim, Yong-Moo Lee, Young Ku, and Yang-Jo Seol. 2021. "Periodontal Wound Healing and Tissue Regeneration: A Narrative Review" Pharmaceuticals 14, no. 5: 456. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14050456






