In Vitro Time-Kill of Common Ocular Pathogens with Besifloxacin Alone and in Combination with Benzalkonium Chloride
Abstract
:1. Introduction
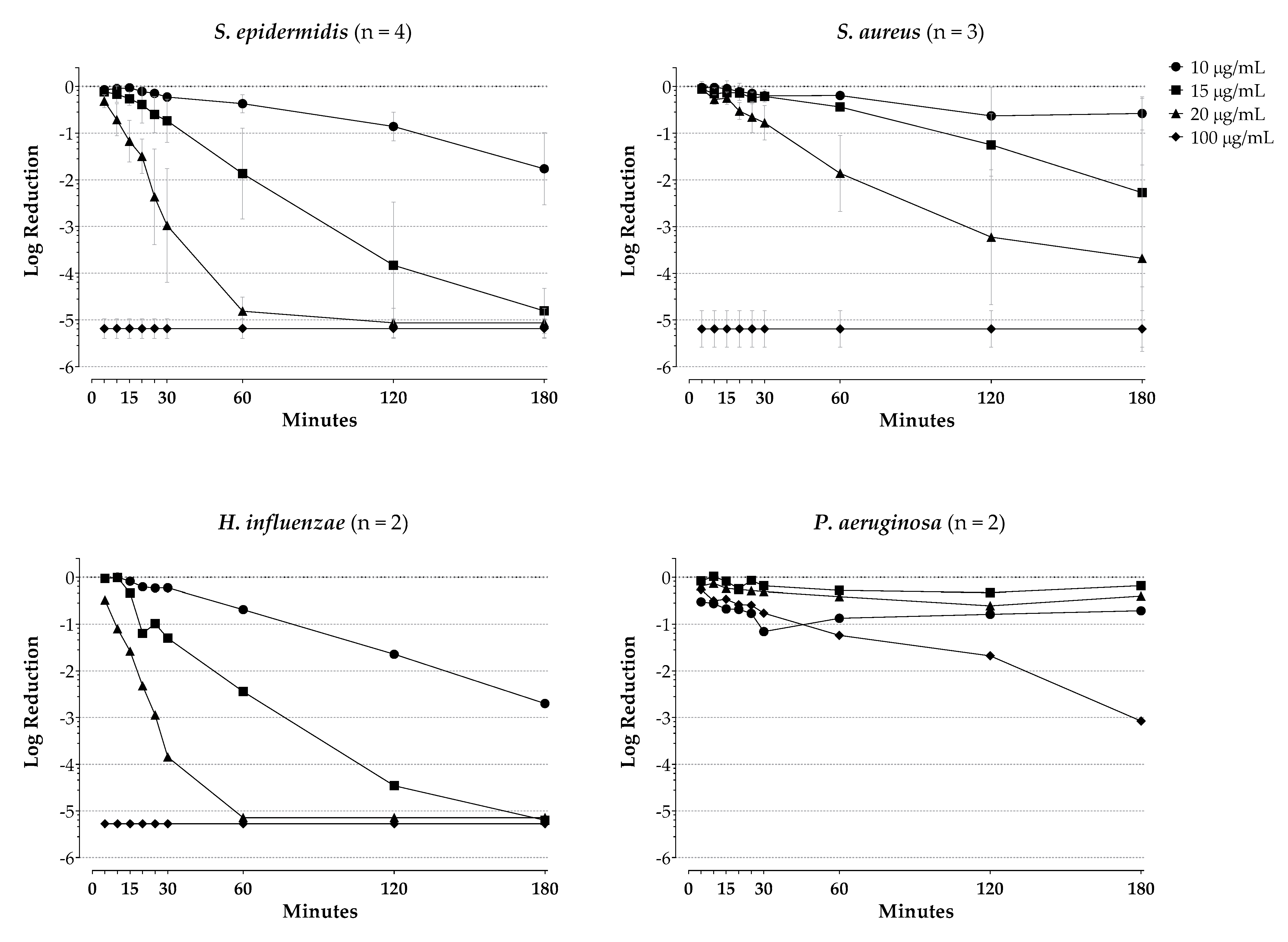
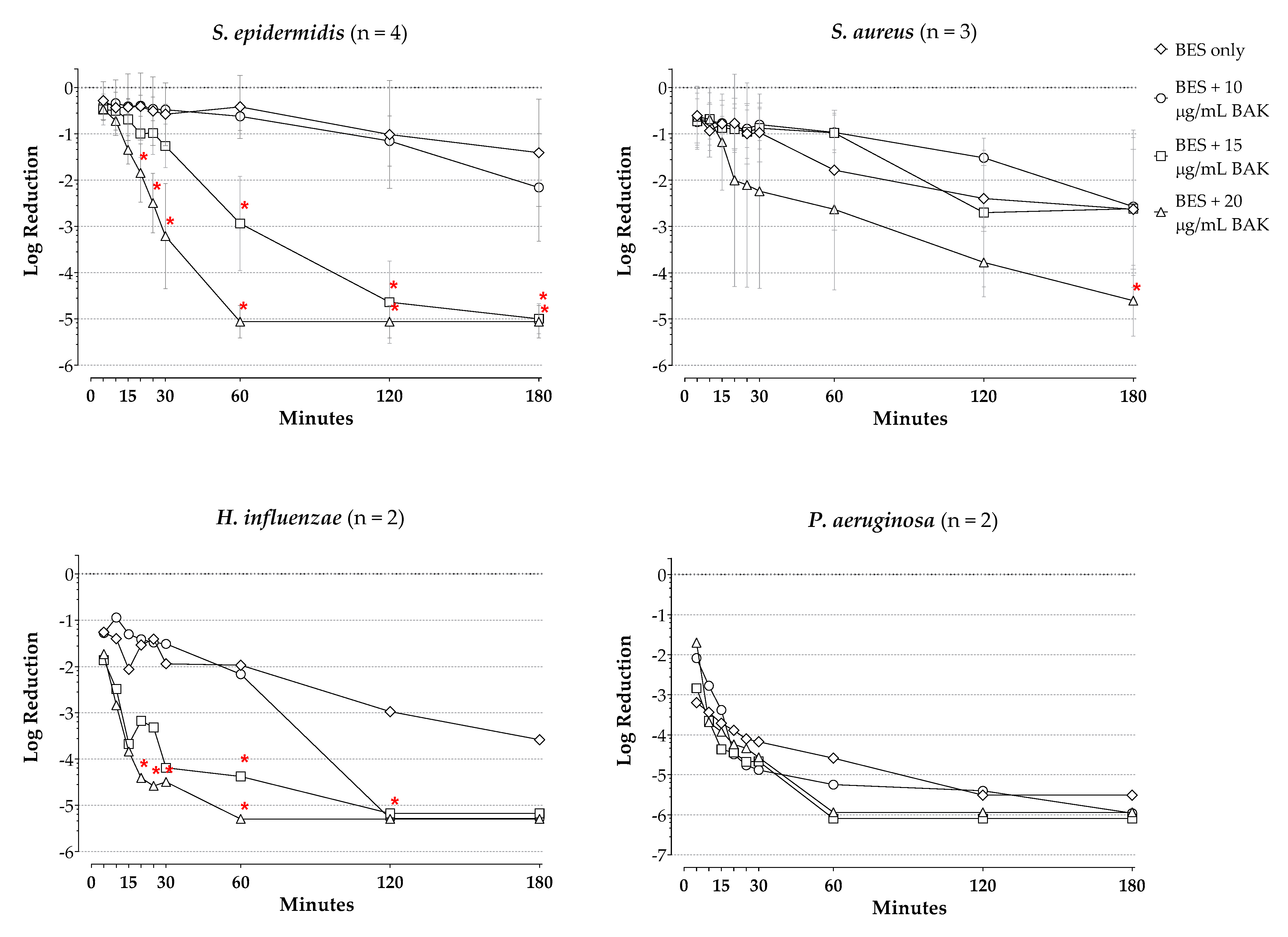
2. Results
3. Discussion
4. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, S.L.; Neuhauser, M.M.; McKinnon, P.S. Quinolones. Antimicrobe. Available online: http://www.antimicrobe.org/d17.asp#t4 (accessed on 2 June 2020).
- Besivance® Prescribing Information; Bausch + Lomb: Bridgewater, NJ, USA, 2020.
- Haas, W.; Pillar, C.M.; Zurenko, G.E.; Lee, J.C.; Brunner, L.S.; Morris, T.W. Besifloxacin, a Novel Fluoroquinolone, Has Broad-Spectrum In Vitro Activity against Aerobic and Anaerobic Bacteria. Antimicrob. Agents Chemother. 2009, 53, 3552–3560. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.; Chang, J.; Flynn, H.W.; Alfonso, E.C. Comparative In Vitro Susceptibility of Besifloxacin and Seven Comparators Against Ciprofloxacin- and Methicillin-Susceptible/Nonsusceptible Staphylococci. J. Ocul. Pharmacol. Ther. 2013, 29, 339–344. [Google Scholar] [CrossRef]
- Asbell, P.A.; Sanfilippo, C.M.; Sahm, D.F.; DeCory, H.H. Trends in Antibiotic Resistance Among Ocular Microorganisms in the United States From 2009 to 2018. JAMA. Ophthalmol. 2020, 138, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, W.; Pillar, C.M.; Hesje, C.K.; Sanfilippo, C.M.; Morris, T.W. In Vitro Time-Kill Experiments with Besifloxacin, Moxifloxacin and Gatifloxacin in the Absence and Presence of Benzalkonium Chloride. J. Antimicrob. Chemother. 2011, 66, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Karpecki, P.; DePaolis, M.; Hunter, J.A.; White, E.M.; Rigel, L.; Brunner, L.S.; Usner, D.W.; Paterno, M.R.; Comstock, T.L. Besifloxacin Ophthalmic Suspension 0.6% in Patients with Bacterial Conjunctivitis: A Multicenter, Prospective, Randomized, Double-Masked, Vehicle-Controlled, 5-Day Efficacy and Safety Study. Clin. Ther. 2009, 31, 514–526. [Google Scholar] [CrossRef]
- Tepedino, M.E.; Heller, W.H.; Usner, D.W.; Brunner, L.S.; Morris, T.W.; Haas, W.; Paterno, M.R.; Comstock, T.L. Phase III Efficacy and Safety Study of Besifloxacin Ophthalmic Suspension 0.6% in the Treatment of Bacterial Conjunctivitis. Curr. Med. Res. Opin. 2009, 25, 1159–1169. [Google Scholar] [CrossRef]
- McDonald, M.B.; Protzko, E.E.; Brunner, L.S.; Morris, T.W.; Haas, W.; Paterno, M.R.; Comstock, T.L.; Usner, D.W. Efficacy and Safety of Besifloxacin Ophthalmic Suspension 0.6% Compared with Moxifloxacin Ophthalmic Solution 0.5% for Treating Bacterial Conjunctivitis. Ophthalmology 2009, 116, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, J.; Silverstein, B.E.; Allaire, C.; Gearinger, L.S.; Bateman, K.M.; Morris, T.W.; Comstock, T.L. Besifloxacin Ophthalmic Suspension 0.6% Administered Twice Daily for 3 Days in the Treatment of Bacterial Conjunctivitis in Adults and Children. Clin. Drug Investig. 2012, 32, 303–317. [Google Scholar] [CrossRef]
- Sanfilippo, C.M.; Allaire, C.M.; DeCory, H.H. Besifloxacin Ophthalmic Suspension 0.6% Compared with Gatifloxacin Ophthalmic Solution 0.3% for the Treatment of Bacterial Conjunctivitis in Neonates. Drugs R D 2017, 17, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, P.; Mathur, U.; Sony, P.; Tandon, R.; Morris, T.W.; Comstock, T.L. Clinical and Antibacterial Efficacy and Safety of Besifloxacin Ophthalmic Suspension Compared with Moxifloxacin Ophthalmic Solution. Asia Pac. J. Ophthalmol. (Phila). 2015, 4, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, B.E.; Allaire, C.; Bateman, K.M.; Gearinger, L.S.; Morris, T.W.; Comstock, T.L. Efficacy and Tolerability of Besifloxacin Ophthalmic Suspension 0.6% Administered Twice Daily for 3 Days in the Treatment of Bacterial Conjunctivitis: A Multicenter, Randomized, Double-Masked, Vehicle-Controlled, Parallel-Group Study in Adults and Children. Clin. Ther. 2011, 33, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, B.E.; Morris, T.W.; Gearinger, L.S.; DeCory, H.H.; Comstock, T.L. Besifloxacin Ophthalmic Suspension 0.6% in the Treatment of Bacterial Conjunctivitis Patients with Pseudomonas aeruginosa Infections. Clin. Ophthalmol. 2012, 6, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Bucci, J.F.; Evans, R.E.; Amico, L.M.; Morris, T.W.; Fluet, A.T.; Sanfilippo, C.M.; DeCory, H.H.; Comstock, T.L. Antibacterial Efficacy of Prophylactic Besifloxacin 0.6% and Moxifloxacin 0.5% in Patients Undergoing Cataract Surgery. Clin. Ophthalmol. 2015, 9, 843–852. [Google Scholar] [CrossRef] [Green Version]
- John, G. A Comparative Study in the Clinical and Microbial Efficacy of Topical Besifloxacin Ophthalmic Suspension 0.6% with Erythromycin Ophthalmic Ointment 0.5% for Management of Acute Blepharitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6263. [Google Scholar]
- Kissling, R.F.; Sanfilippo, C.M.; DeCory, H.H. Comparative In Vitro Activity of Antibiotics Frequently Used in the Management of Staphylococcal Blepharitis. In Proceedings of the Association for Research in Vision and Ophthalmology Annual Meeting (ARVO), Digital, 7 May 2021. [Google Scholar]
- Tu, Y.; Boschert, B.; Schwab, J.; Wagner, R.; DeRespinis, P.; Guo, S. Management of Congenital Nasolacrimal Duct Obstruction with Infection in Infants using Besifloxacin-A Prospective Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4870. [Google Scholar]
- Tu, Y.; Boschert, K.; Schwab, J.V.; Wagner, R.S.; Derespinis, P.; Guo, S. Comparison of Besifloxacin and Polymyxin–trimethoprim in Treating Congenital Nasolacrimal Duct Obstruction with Infection in Children. In Proceedings of the American Society of Cataract and Refractive Surgery Annual Symposium and Congress (ASCRS), San Diego, CA, USA, 17 April 2015. [Google Scholar]
- Freeman, P.D.; Kahook, M.Y. Preservatives in Topical Ophthalmic Medications: Historical and Clinical Perspectives. Expert Rev. Ophthalmol. 2009, 4, 59–64. [Google Scholar] [CrossRef]
- Saji, M.; Usuki, R.; Ibaraki, N.; Hayama, N.; Osono, E.; Ohkuni, H. Studies of Antibacterial Activity of Benzalkonium Chloride as Preservative for Ophthalmic Solutions Against Gram-positive Cocci and Negative Rods. Jpn. J. Pharm. Health Care Sci. 2003, 29, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Block, S.S. (Ed.) Disinfection, Sterilization and Preservation, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1991; p. 633. [Google Scholar]
- Sheikh, A.; Hurwitz, B.; Van Schayck, C.P.; McLean, S.; Nurmatov, U. Antibiotics Versus Placebo for Acute Bacterial Conjunctivitis. Cochrane Database Syst. Rev. 2012, 9, CD001211. [Google Scholar] [CrossRef] [Green Version]
- Hesje, C.K.; Borsos, S.D.; Blondeau, J.M. Benzalkonium Chloride Enhances Antibacterial Activity of Gatifloxacin and Reduces its Propensity to Select for Fluoroquinolone-Resistant Strains. J. Ocul. Pharmacol. Ther. 2009, 25, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.; Borsos, S.; Hesje, C. Antimicrobial Efficacy of Gatifloxacin and Moxifloxacin with and without Benzalkonium Chloride Compared with Ciprofloxacin and Levofloxacin Against Methicillin-resistant Staphylococcus aureus. J. Chemother. 2007, 19, 146–151. [Google Scholar] [CrossRef]
- Stratton, C.W. Dead Bugs Don’t Mutate: Susceptibility Issues in the Emergence of Bacterial Resistance. Emerg. Infect. Dis. 2003, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dean, N.C. Encouraging News from the Antibiotic Resistance Front. Chest 2003, 124, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.P.; Kowalski, B.R.; Romanowski, E.G.; Mah, F.S.; Thompson, P.P.; Gordon, Y.J. The In vitro Impact of Moxifloxacin and Gatifloxacin Concentration (0.5% vs. 0.3%) and the Addition of Benzalkonium Chloride on Antibacterial Efficacy. Am. J. Ophthalmol. 2006, 142, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hatt, J.K.; Weigand, M.R.; Krishnan, R.; Pavlostathis, S.G.; Konstantinidis, K.T. Genomic and Transcriptomic Insights into How Bacteria Withstand High Concentrations of Benzalkonium Chloride Biocides. Appl. Environ. Microbiol. 2018, 84, e00197-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, R.; Joynson, J.; Forbes, B. The Relationships and Susceptibilities of Some Industrial, Laboratory and Clinical Isolates of Pseudomonas Aeruginosa to Some Antibiotics and Biocides. J. Appl. Microbiol. 2001, 91, 972–984. [Google Scholar] [CrossRef] [Green Version]
- Hyon, J.Y.; Eser, I.; O’Brien, T.P. Kill Rates of Preserved and Preservative-Free Topical 8-Methoxy Fluoroquinolones Against Various Strains of Staphylococcus. J. Cataract. Refract. Surg. 2009, 35, 1609–1613. [Google Scholar] [CrossRef]
- Proksch, J.W.; Granvil, C.P.; Siou-Mermet, R.; Comstock, T.L.; Paterno, M.R.; Ward, K.W. Ocular Pharmacokinetics of Besifloxacin Following Topical Administration to Rabbits, Monkeys, and Humans. J. Ocul. Pharmacol. Ther. 2009, 25, 335–344. [Google Scholar] [CrossRef]
- Friedlaender, M.H.; Breshears, D.; Amoozgar, B.; Sheardown, H.; Senchyna, M. The Dilution of Benzalkonium Chloride (BAK) in the Tear Film. Adv. Ther. 2006, 23, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Colby, K.A.; Deng, S.; McDonnell, P.; Meisler, D.M.; Raizman, M.B.; Sheppard, J.D.; Sahm, D.F. Ocular TRUST: Nationwide Antimicrobial Susceptibility Patterns in Ocular Isolates. Am. J. Ophthalmol. 2008, 145, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Oydanich, M.; Dingle, T.; Hamula, C.L.; Ghisa, C.; Asbell, P. Retrospective Report of Antimicrobial Susceptibility Observed in Bacterial Pathogens Isolated from Ocular Samples at Mount Sinai Hospital, 2010 to 2015. Antimicrob. Resist. Infect. Control. 2017, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Miller, D. Update on the Epidemiology and Antibiotic Resistance of Ocular Infections. Middle East Afr. J. Ophthalmol. 2017, 24, 30–42. [Google Scholar]
- Gentile, R.C.; Shukla, S.; Shah, M.; Ritterband, D.C.; Engelbert, M.; Davis, A.; Hu, D.-N. Microbiological Spectrum and Antibiotic Sensitivity in Endophthalmitis: A 25-year review. Ophthalmology 2014, 121, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial Profile of Ocular Infections: A Systematic Review. BMC. Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Vola, M.M.; Moriyama, A.S.; Lisboa, R.; Hirai, F.E.; Bispo, P.J.M.; Höfling-Lima, A.L. Prevalence and Antibiotic Susceptibility of Methicillin-Resistant Staphylococcus Aureus in Ocular Infections. Arq. Bras. Oftalmol. 2013, 76, 350–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.; Pershing, S.; Walvick, M.; Tanaka, S. Trends in Ophthalmic Manifestations of Methicillin-Resistant Staphylococcus Aureus (MRSA) in a Northern California Pediatric Population. J. AAPOS. 2013, 17, 243–247. [Google Scholar] [CrossRef]
- Asbell, P.A.; Sahm, D.F.; Shaw, M.; Draghi, D.C.; Brown, N.P. Increasing Prevalence of Methicillin Resistance in Serious Ocular Infections Caused by Staphylococcus Aureus in the United States: 2000 to 2005. J. Cataract Refract. Surg. 2008, 34, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Chu, H.-S.; Wang, I.-J.; Chen, W.-L.; Hu, F.-R. Microbial Keratitis in Taiwan: A 20-Year Update. Am. J. Ophthalmol. 2019, 205, 74–81. [Google Scholar] [CrossRef]
- Chang, V.S.; Dhaliwal, D.K.; Raju, L.; Kowalski, R.P. Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis. Cornea 2015, 34, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondeau, J.M.; Hansen, G.T.; Metzler, K.L.; Borsos, S.; Chau, J. Optimal Killing of Streptococcus Pneumoniae by Gemifloxacin, Levofloxacin and Moxifloxacin. Round Table Ser. R. Soc. Med. 2002, 76, 15–26. [Google Scholar]
- Blondeau, J.; Blondeau, L.; Hesje, C.; Borsos, S. Application of Two Methods to Determine Killing of Streptococcus pneumoniae by Various Fluoroquinolones. J. Chemother. 2006, 18, 366–372. [Google Scholar] [CrossRef]
- Blondeau, J.; Borsos, S.; Blondeau, L.; Blondeau, B. In Vitro Killing of Escherichia Coli, Staphylococcus Pseudintermedius and Pseudomonas Aeruginosa by Enrofloxacin in Combination with its Active Metabolite Ciprofloxacin Using Clinically Relevant Drug Concentrations in the Dog and Cat. Vet. Microbiol. 2012, 155, 284–290. [Google Scholar] [CrossRef] [PubMed]

| BES (100 µg/mL) | BES a + BAK (10 µg/mL) | BES a + BAK (15 µg/mL) | BES a + BAK (20 µg/mL) | |
|---|---|---|---|---|
| S. epidermidis, n = 4 | ||||
| Mean Δlog CFU/mL | −1.41 | −2.16 | −5.00 | −5.06 |
| Mean Difference (95% CI) | 0.753 (−0.306, 1.81) | 3.59 (2.53, 4.65) | 3.66 (2.60, 4.71) | |
| p-value b | NS | <0.0001 | <0.0001 | |
| CipR MRSE, n = 1 | ||||
| Δlog CFU/mL | −0.350 | −0.500 | −4.81 | −4.81 |
| Mean Difference (95% CI) | 0.150 (−3.43, 3.73) | 4.46 (0.876, 8.04) | 4.49 (0.906, 8.07) | |
| p-value b | NS | 0.0092 | 0.0086 | |
| CipS MRSE, n = 2 | ||||
| Mean Δlog CFU/mL | −2.41 | −2.96 | −5.24 | −5.32 |
| Mean Difference (95% CI) | 0.545 (−0.463, 1.55) | 2.83 (1.82, 3.84) | 2.91 (1.90, 3.92) | |
| p-value b | NS | <0.0001 | <0.0001 | |
| CipR MSSE, n = 1 | ||||
| Δlog CFU/mL | −0.460 | −2.23 | −4.70 | −4.77 |
| Mean Difference (95% CI) | 1.77 (−1.71, 5.25) | 4.24 (0.765, 7.72) | 4.31 (0.835, 7.79) | |
| p-value b | NS | 0.0110 | 0.0095 | |
| S. aureus, n = 3 | ||||
| Mean Δlog CFU/mL | −2.63 | −2.57 | −2.62 | −4.60 |
| Mean Difference (95% CI) | −0.0567 (−1.81, 1.70) | −0.0100 (−1.77, 1.75) | 1.98 (0.221, 3.73) | |
| p-value b | NS | NS | 0.0196 | |
| CipR MRSA, n = 1 | ||||
| Δlog CFU/mL | −1.15 | −1.67 | −1.31 | −5.35 |
| Mean Difference (95% CI) | 0.520 (−1.96, 3.00) | 0.160 (−2.32, 2.64) | 4.20 (1.72, 6.68) | |
| p-value b | NS | NS | 0.0003 | |
| CipR MSSA, n = 1 | ||||
| Δlog CFU/mL | −3.58 | −1.75 | −2.00 | −3.82 |
| Mean Difference (95% CI) | −1.83 (−3.37, −0.29) | −1.58 (−3.12, −0.04) | 0.024 (−1.30, 1.78) | |
| p-value b | 0.0141 | 0.0427 | NS | |
| CipS MSSA, n = 1 | ||||
| Δlog CFU/mL | −3.15 | −4.29 | −4.54 | −4.64 |
| Mean Difference (95% CI) | 1.14 (−2.05, 4.33) | 1.39 (−1.80, 4.58) | 1.49 (−1.70, 4.68) | |
| p-value b | NS | NS | NS | |
| H. influenzae, n = 2 | ||||
| Mean Δlog CFU/mL | −3.58 | −5.29 | −5.18 | −5.30 |
| Mean Difference (95% CI) | 1.71 (−0.380, 3.79) | 1.60 (−0.490, 3.68) | 1.72 (−0.370, 3.80) | |
| p-value b | NS | NS | NS | |
| P. aeruginosa, n = 2 | ||||
| Mean Δlog CFU/mL | −5.51 | −5.96 | −6.10 | −5.94 |
| Mean Difference (95% CI) | 0.450 (−2.27, 3.17) | 0.585 (−2.13, 3.30) | 0.430 (−2.29, 3.15) | |
| p-value b | NS | NS | NS | |
| CipR P. aeruginosa, n = 1 | ||||
| Δlog CFU/mL | −5.63 | −5.93 | −5.99 | −5.97 |
| Mean Difference (95% CI) | 0.300 (−2.86, 3.46) | 0.360 (−2.80, 3.52) | 0.340 (−2.82, 3.50) | |
| p-value b | NS | NS | NS | |
| CipS P. aeruginosa, n = 1 | ||||
| Δlog CFU/mL | −5.39 | −5.99 | −6.20 | −5.91 |
| Mean Difference (95% CI) | 0.600 (−1.77, 2.97) | 0.810 (−1.56, 3.18) | 0.520 (−1.85, 2.89) | |
| p-value b | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blondeau, J.; DeCory, H. In Vitro Time-Kill of Common Ocular Pathogens with Besifloxacin Alone and in Combination with Benzalkonium Chloride. Pharmaceuticals 2021, 14, 517. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060517
Blondeau J, DeCory H. In Vitro Time-Kill of Common Ocular Pathogens with Besifloxacin Alone and in Combination with Benzalkonium Chloride. Pharmaceuticals. 2021; 14(6):517. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060517
Chicago/Turabian StyleBlondeau, Joseph, and Heleen DeCory. 2021. "In Vitro Time-Kill of Common Ocular Pathogens with Besifloxacin Alone and in Combination with Benzalkonium Chloride" Pharmaceuticals 14, no. 6: 517. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060517





