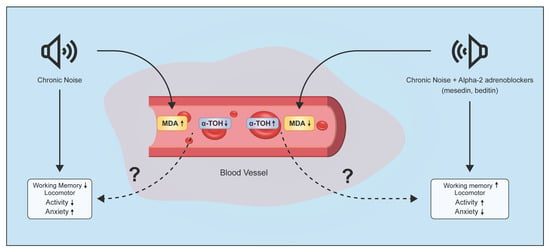
Alpha2-Adrenoblockers Regulate Development of Oxidative Stress and Cognitive Behaviour of Rats under Chronic Acoustic Stress Conditions
Abstract
:1. Introduction
2. Results
2.1. Biochemical Findings
2.1.1. Changes of MDA Level in Plasma and EM under Chronic Acoustic Stress Conditions and Use of α2-Adrenoblockers
2.1.2. Changes of α-T in Plasma and EM under Chronic Acoustic Stress Conditions and Use of α2-Adrenoblockers
2.2. Behavioural Findings
2.2.1. Quantification of Arm Entries in Y-Maze
2.2.2. Spontaneous Alternations
2.2.3. Total Mobile Episodes
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Purification of Erythrocyte Membrane
4.3. Determination of MDA
4.4. Determination of α-T Content in Blood Plasma and EM
4.5. Y-Maze
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidman, M.; Standring, R. Noise and quality of life. Int. J. Envrion. Res. Public Health 2010, 7, 3730–3738. [Google Scholar] [CrossRef] [Green Version]
- Bogden, J.D. Detrimental health effects of noise pollution. J. Med. Soc. 2006, 71, 847–851. [Google Scholar]
- Daiber, A.; Kröller-Schön, S.; Oelze, M.; Hahad, O.; Li, H.; Schulz, R.; Steven, S.; Münzel, T. Oxidative stress and inflammation contribute to traffic noise-induced vascular and cerebral dysfunction via uncoupling of nitric oxide synthases. Redox Biol. 2020, 34, 101506. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Stefania, S.; Vincent, J.; Luigia, T.; Karl-Heinz, K. Severe Life Stress and Oxidative Stress in the Brain:From Animal Models to Human Pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef] [Green Version]
- Dubinina, E.E. Oxygine Metabolism Products in the Functional Activity of Cells (Life and Death, Creation and Destruction). Physiological and Clinical-Biochemical Aspects; Medical Press: St. Petersburg, Russia, 2006. (In Russia) [Google Scholar]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Dündar, Y.; Aslan, R. Antioxidative stress. East. J. Med. 2000, 5, 45–47. [Google Scholar]
- Melkonyan, M.M.; Shirinyan, E.A.; Hynanyan, L.S.; Manukyan, A.L.; Minasyan, A.A.; Hakobyan, N.R.; Yavroyan, J.V. The effects of selective alpha-adrenoblocker beditin on the intensity of lipid peroxidation and membrane phosphoinositides content in acoustic stress conditions. New Armen. Med. J. 2010, 4, 15–24. [Google Scholar]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 761264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirinyan, E.; Harutyunyan, S. Mesedin—A new anti-hypoxic property possessing peripheral post-synaptic α2-Adrenoblocker. Med. Sci. Educ. 2015, 18, 27–31. [Google Scholar]
- Westman, J.C.; Walters, J. Noise and stress: A comprehensive approach. Envrion. Health Perspect. 1981, 41, 291–309. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R. Hormones, brain and stress. Endocr. Regul. 2003, 37, 51–68. [Google Scholar]
- Arnsten, F.T.; Goldman-Rakic, P.S. Noise stress impairs prefrontal cognitive function in monkeys. Arch. Gen. Psychiatry 1998, 55, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.; Schmauss, C. Strain-Specific Cognitive Deficits in Adult Mice Exposed to Early Life Stress. Behav. Neurosci. 2011, 125, 29–36. [Google Scholar] [CrossRef]
- Jessica, C.J.; Katy, S.; Alexander, R.G.; Victor, M.L.; Jeremy, S.B.; Gokhan, O.; Pengcheng, Z.; Samantha, K.O.; Matthew, A.W. Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron 2018, 97, 670–683. [Google Scholar]
- Cleal, M.; Fontana, B.D.; Ranson, D.C.; McBride, S.D.; Swinny, J.D.; Redhead, E.S.; Parker, M.O. The Free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behav. Res. 2021, 53, 536–557. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, A.L.; Grigoryan, A.S.; Hunanyan, L.S.; Harutyunyan, H.A.; Manukyan, M.V.; Mkrtchyan, V.S.; Melkonyan, M.M. Alfa2-adrenoblockers attenuate the elevated plasma cholesterol, anxiety levels and restore impaired spatial memory of rats under the chronic noise exposure. Sci. Total Environ. 2020, 740. [Google Scholar] [CrossRef]
- Babisch, W. Stress hormones in the research on cardiovascular effects of noise. Noise Health 2003, 5, 1–11. [Google Scholar]
- Manikandan, S.; Padma, M.K.; Srikumar, R.; Jeya, P.N.; Muthuvel, A.; Sheela, D.R. Effect of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci. Lett. 2006, 399, 17–22. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetoni, A.R.; Ralli, M.; Sergi, B.; Parrilla, C.; Troiani, D.; Paludetti, G. Protective properties of antioxidant drugs in noise-induced hearing loss in the guinea pig. Audiol. Med. 2006, 6, 271–277. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R.S. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2525967. [Google Scholar] [CrossRef]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and inflammation: Possible effects on immunity, chronic diseases, andlifespan. J. Am. Coll. Nutr. 2015, 34, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Mueller, C.; Zabel, M.; Croffon, A.; Kirsch, W.M.; Ghribi, O.; Squitti, R.; Perry, G. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol. Dis. 2013, 59, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rasheed, O.F.; Farid, Y.Y.; Al-Nasiri, U.S. Coenzyme Q10 and oxidative stress markers in seminal plasma of Iraqi patients with male infertility. Saudi Med. J. 2010, 31, 501–506. [Google Scholar] [PubMed]
- Panin, L.E.; Mokrushnikov, P.V.; Kunitsyn, V.G.; Zaitsev, B.N.; Zaitsev, B.N. Interaction Mechanism of Cortisol and Catecholamines with Structural Components of Erythrocyte Membrane. J. Phys. Chem. B 2010, 114, 9462–9473. [Google Scholar] [CrossRef]
- Mokrushnikov, P.V.; Panin, L.E. The action of stress hormones on the structure and function of erythrocyte membrane. Gen. Physiol. Biophys. 2015, 34, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Kunitsyn, V.G.; Panin, L.E. Mechanism of Erythrocyte Deformation under the Action of Stress Hormones. Int. J. Biophys. 2013, 3, 1–14. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Delmas-Beauvieux, M.C.; Peuchant, E.; Richard-Harston, S.; Decamps, A.; Reignier, B.; Emeriau, J.P.; Rainfray, M. Antioxidant defenses and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 2001, 30, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghajanov, M.; Chavushyan, V.; Matinyan, S.; Danielyan, M.; Yenkoyan, K. Alzheimer’s disease-like pathology-triggered oxidative stress, alterations in monoamines levels, and structural damage of locus coeruleus neurons are partially recovered by a mix of proteoglycans of embryonic genesis. Neurochem. Int. 2019, 131, 104531. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman., H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podda, M.; Grundmann-Kollmann, M. Low molecular weight antioxidants and their role in skin ageing. Clin. Exp. Dermatol. 2001, 26, 578–582. [Google Scholar] [CrossRef]
- Rossato, M.F.; Hoffmeister, C.; Tonello, R.; de Oliveira Ferreira, A.P.; Ferreira, J. Anti-inflammatory effects of vitamin E on adjuvant-induced arthritis in rats. Inflammation 2015, 38, 606–615. [Google Scholar] [CrossRef]
- Azzi, A. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef]
- Atkinson, J.; Harrainne, T.; Wassall, S.R.; Stilwell, W.; Katsaras, J. The location and behavior of alpha-tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Melkonian, M.M. The changes of the Erythrocyte Membranes Lipids Fatty Acids composition under the acoustic stress Conditions and a-tocopherolacetate Application. Med. Sci. Armen. 1993, 33, 71–76. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Manukyan, A.L.; Grigoryan, A.S.; Hunanyan, L.S.; Harutyunyan, H.A.; Manukyan, M.V.; Melkonyan, M.M. Adrenergic alpha-2 receptor antagonists cease augmented oxidation of plasma proteins and anxiety of rats caused by chronic noise exposure. Noise Health 2020, 22, 63–69. [Google Scholar] [CrossRef]
- Manukyan, A. Alfa-2 adrenoblokers decrease elevated carbonylation of erythrocytes’ membranes proteins and regulate behavioral changes induced by noise action. Life Sci. 2020, 246. [Google Scholar] [CrossRef]
- Cleva, V.; Robert, D.K. Antioxidant-Induced Stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef] [Green Version]
- Moret, C.; Briley, M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011, 7, 9–13. [Google Scholar]
- Ruslán, Á.D.; Annia, G. Adrenaline and Noradrenaline: Protectors against Oxidative Stress or Molecular Targets? J. Phys. Chem. 2015, 119, 3479–3491. [Google Scholar] [CrossRef]
- Langer, S.Z. α2—Adrenoceptors in the treatment of major neuropsychiatric disorders. Trends Pharmacol. Sci. 2015, 38, 196–202. [Google Scholar] [CrossRef]
- Kable, J.W.; Murrin, L.C.; Bylund, D.B. In vivo gene modification elucidates subtype-specific functions of alpha (2)-adrenergic receptors. J. Pharmacol. Exp. 2000, 293, 1–7. [Google Scholar]
- Frankenhaeuser, M. Psychoneuroendocrine approaches to the study of emotion as related to stress on coping. In Nebraska Symposium on Motivation; Howe, H.E., Dienstbier, R.A., Eds.; University of Nebraska Press: Lincoln, NE, USA, 1979. [Google Scholar]
- Howard, E. Hippocampus: Cognitive Processes and Neural Representations that Underlie Declarative Memory. Neuron 2004, 44, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Tannenholz, L.; Jimenez, J.C.; Kheirbek, M.A. Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Front. Behav. Neurosci. 2014, 8, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parfitt, G.M.; Nguyen, R.; Bang, J.Y.; Aqrabawi, A.J.; Tran, M.M.; Seo, D.; Richards, B.A.; Kim, J.C. Bidirectional control of anxiety-related behaviors in mice: Role of inputs arising from the ventral hippocampus to the lateral septum and medial prefrontal cortex. Neuropsychopharmacology 2017, 42, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.C.; Singer, J.E. Urban Stress: Experiments on Noise and Social Stressors; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Bücheler, M.M.; Hadamek, K.; Hein, L. Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience 2002, 109, 819–826. [Google Scholar] [CrossRef]
- Li, I.-H.; Shih, J.-H.; Jhao, Y.-T.; Chen, H.-C.; Chiu, C.-H.; Chen, C.-F.F.; Huang, Y.-S.; Shiue, C.-Y.; Ma, K.-H. Regulation of noise-induced loss of serotonin transporters with resveratrol in a rat model using 4-[18F]-ADAM/small-animal positron emission tomography. Molecules 2019, 24, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, R.; Sheela, D.R.; James, S.; Manohar, S. Noise-stress-induced brain neurotransmitter changes and the effect of ocimum sanctum (Linn) treatment in albinorats. J. Pharm. Sci. 2005, 98, 354–360. [Google Scholar] [CrossRef]
- Ursino, M.G.; Vasina, V.; Raschi, E.; Crema, F.; De Ponti, F. The β3-adrenoceptor as a therapeutic target: Current perspectives. Pharm. Res. 2009, 59, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Rodrigeuz Manzanares, P.A.; Isoardi, N.A.; Carrer, H.F.; Malina, V.A. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amigdala. J. Neurosci. 2005, 25, 8725–8734. [Google Scholar] [CrossRef]
- Emily, P.; Nafisa, J. Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio Protoc. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Melkonyan, M.M.; Hunanyan, L.; Lourhmati, A.; Layer, N.; Beer-Hammer, S.; Yenkoyan, K.; Schwab, M.; Danielyan, L. Neuroprotective, neurogenic, and amyloid beta reducing effect of a novel alpha 2-Adrenoblocker, mesedin, on astroglia and neuronal progenitors upon hypoxia and glutamate exposure. Int. J. Mol. Sci. 2018, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Melkonyan, M.M.; Melik-Agaeva, E.; Mchitaryan, V.G. The intensity of lipid peroxidation processes and the activity of enzymes depending on sex under stress. Med. Sci. Armen. 1986, 26, 322–328. (In Russia) [Google Scholar]
- Yenkoyan, K.; Fereshetyan, K.; Matinyan, S.; Chavushyan, V.; Aghajanov., M. The role of monoamines in the development of Alzheimer’s disease and neuroprotective effect of a proline rich polypeptide. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 76–82. [Google Scholar] [CrossRef]
- Di, G.Q.; Qin, Z.Q. Influence of combined traffic noise on the ability of learning and memory. Noise Health 2018, 20, 9–15. [Google Scholar]
- Low, T.Y.; Seow, T.K.; Chung, M.C. Separation of human erythrocyte membrane associated proteins with one-dimensional and two-dimensional gel electrophoresis followed by identification with matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Proteomics 2002, 2, 1229–1239. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzym. 1990, 186, 407–421. [Google Scholar]
- Duggan, D.D. Spectrofluorometric determination of tocopherols. Arch. Biochem. Biophys. 1954, 84, 116–126. [Google Scholar] [CrossRef]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Page, D.T.; Merzlyak, I.; Kim, C.; Tecott, L.H.; Janak, P.H.; Rubenstein, J.L.; Sur, M. Reduced conditioned fear response in mice that lack Dlx1 and show subtype-specific loss of interneurons. J. Neurodev. Disord. 2009, 1, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melkonyan, M.; Manukyan, A.; Hunanyan, L.; Grigoryan, A.; Harutyunyan, H.; Sukiasyan, L.; Danielyan, L.; Yenkoyan, K. Alpha2-Adrenoblockers Regulate Development of Oxidative Stress and Cognitive Behaviour of Rats under Chronic Acoustic Stress Conditions. Pharmaceuticals 2021, 14, 529. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060529
Melkonyan M, Manukyan A, Hunanyan L, Grigoryan A, Harutyunyan H, Sukiasyan L, Danielyan L, Yenkoyan K. Alpha2-Adrenoblockers Regulate Development of Oxidative Stress and Cognitive Behaviour of Rats under Chronic Acoustic Stress Conditions. Pharmaceuticals. 2021; 14(6):529. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060529
Chicago/Turabian StyleMelkonyan, Magdalina, Ashkhen Manukyan, Lilit Hunanyan, Artem Grigoryan, Hayk Harutyunyan, Lilit Sukiasyan, Lusine Danielyan, and Konstantin Yenkoyan. 2021. "Alpha2-Adrenoblockers Regulate Development of Oxidative Stress and Cognitive Behaviour of Rats under Chronic Acoustic Stress Conditions" Pharmaceuticals 14, no. 6: 529. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060529