Safety and Benefits of Contraceptives Implants: A Systematic Review
Abstract
:1. Introduction
2. Evidence Acquisition
3. Results
3.1. Levonorgestrel Implant
3.2. Etonogestrel Implant
3.2.1. Implanon
3.2.2. Nexplanon
3.2.3. ENG Implant Extended Use above Three Years
3.3. Contraceptive Implant in Adolescent Girls
3.4. Contraceptive Implant Placement in Post-Partum/Post-Abortion Women
3.4.1. Post-Partum Implant Placement
3.4.2. Post-Abortion Implant Placement
3.5. Non-Contraceptive Use of ENG Implant
3.5.1. Endometriosis
3.5.2. Endometrial Action
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahamondes, L.; Fernandes, A.; Monteiro, I.; Bahamondes, M.V. Long-acting reversible contraceptive (LARCs) methods. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 66, 28–40. [Google Scholar] [CrossRef]
- Hubacher, D.; Spector, H.; Monteith, C.; Chen, P.L.; Hart, C. Long-acting reversible contraceptive acceptability and unintended pregnancy among women presenting for short-acting methods: A randomized patient preference trial. Am. J. Obstet. Gynecol. 2017, 216, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Black, A.; Guilbert, E. Society of Obstetricians and Gynaecologists of Canada. Canadian Contraception Consensus (Part 1 of 4). J. Obstet. Gynaecol. Can. 2015, 37, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Black, A.; Guilbert, E. Canadian Contraception Consensus (Part 2 of 4). J. Obstet. Gynaecol. Can. 2015, 37, 1033–1039. [Google Scholar] [CrossRef]
- Hindy, J.R.; Souaid, T.; Larus, C.T.; Glanville, J.; Aboujaoude, R. Nexplanon migration into a subsegmental branch of the pulmonary artery: A case report and review of the literature. Medicine 2020, 99, e18881. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, H.; Creinin, M.D. The contraceptive implant. Clin. Obstet. Gynecol. 2007, 50, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Peralta, O.; Diaz, S.; Croxatto, H. Subdermal contraceptive implants. J. Steroid Biochem. Mol. Biol. 1995, 53, 223–226. [Google Scholar] [CrossRef]
- Le, J.; Tsourounis, C. Implanon: A critical review. Ann. Pharmacother. 2001, 35, 329–336. [Google Scholar] [CrossRef]
- Darney, P.D. A Hormonal implants: Contraception for a new century. Am. J. Obstet. Gynecol. 1994, 170, 536–543. [Google Scholar] [CrossRef]
- Hatcher, R.A. Contraceptive Technology; Ardent Media: New York, NY, USA, 2011. [Google Scholar]
- Thaxton, L.; Lavelanet, A. Systematic review of efficacy with extending contraceptive implant duration. Int. J. Gynaecol. Obstet. 2019, 44, 2–8. [Google Scholar] [CrossRef]
- Wehrle, K.E. The Norplant System: Easy to insert, easy to remove. Nurse Pract. 1994, 19, 47–54. [Google Scholar] [CrossRef]
- Coukell, A.J.; Balfour, J.A. Levonorgestrel subdermal implants. A review of contraceptive efficacy and acceptability. Drugs 1998, 55, 861–887. [Google Scholar] [CrossRef]
- Lynn, M.M.; Holdcroft, C. New concepts in contraception: Norplant subdermal implant. Nurse Pract. 1992, 17, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ramdhan, R.C.; Simonds, E.; Wilson, C.; Loukas, M.; Oskouian, R.J.; Tubbs, R.S. Complications of Subcutaneous Contraception: A Review. Cureus 2018, 10, e2132. [Google Scholar] [CrossRef] [Green Version]
- International Planned Parenthood Federation IPPF. International Medical Advisory Panel IMAP. IMAP statement on Norplant subdermal contraceptive implant system. IPPF Med. Bull. 1993, 27, 1–3. [Google Scholar]
- Cooper, M. Norplant. Aust. N. Z. J. Obstet. Gynaecol. 1991, 31, 265–272. [Google Scholar] [CrossRef]
- Meirik, O.; Farley, T.; Sivin, I.; Diaz, S. Post-marketing surveillance of Norplant contraceptive implants: I. Contraceptive efficacy and reproductive health. Contraception 2001, 63, 67–86. [Google Scholar]
- Ladipo, O.; Coutinho, E.M. Contraceptive implants. Curr. Opin. Obstet. Gynecol. 1994, 6, 564–569. [Google Scholar] [CrossRef]
- Kirkman, R.J.; Bromham, D.R.; O’Connor, T.P.; Sahota, J.E. Prospective multicentre study comparing levonorgestrel implants with a combined contraceptive pill: Final results. Br. J. Fam. Plann. 1999, 25, 36–40. [Google Scholar]
- Pam, V.C.; Mutihir, J.T.; Nyango, D.D.; Shambe, I.; Egbodo, C.O.; Karshima, J.A. Sociodemographic profiles and use-dynamics of Jadelle (levonorgestrel) implants in Jos, Nigeria. Niger. Med. J. 2016, 57, 314. [Google Scholar] [CrossRef] [PubMed]
- Sivin, I. Contraception with NORPLANT implants. Hum. Reprod. 1994, 9, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.S.; Tiitinen, A.; Affandi, B.; Brache, V.; Croxatto, H.B.; Diaz, S.; Ginsburg, J.; Gu, S.; Holma, P.; Johansson, E. Norplant consensus statement and background review. Contraception 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Sivin, I. Findings in Phase III Studies of Norplant Implants. In Long-Acting Contraceptive Delivery Systems; Zatuchni, G.I., Goldsmith, A., Shelton, J.D., Sciarra, J.J., Eds.; Harper & Row: Philadelphia, PA, USA, 1984. [Google Scholar]
- Hubacher, D.; Lopez, L.; Steiner, M.J.; Dorflinger, L. Menstrual pattern changes from levonorgestrel subdermal implants and DMPA: Systematic review and evidence-based comparisons. Contraception 2009, 80, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aleem, H.; d’Arcangues, C.; Vogelsong, K.M.; Gaffield, M.L.; Gülmezoglu, A.M. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst. Rev. 2013, 10, CD003449. [Google Scholar]
- Matulich, M.C.; Chen, M.J.; Schimmoeller, N.R.; Hsia, J.K.; Uhm, S.; Wilson, M.D.; Creinin, M.D. Referral Center Experience With Nonpalpable Contraceptive Implant Removals. Obstet. Gynecol. 2019, 134, 801–806. [Google Scholar] [CrossRef]
- Marin, R.; McMillian, D. Ulnar neuropathy associated with subdermal contraceptive implant. South. Med. J. 1998, 91, 875–878. [Google Scholar] [CrossRef]
- Smith, J.M.; Conwit, R.A.; Blumenthal, P.D. Ulnar nerve injury associated with removal of Norplant implants. Contraception 1998, 57, 99–101. [Google Scholar] [CrossRef]
- Miller, L.; Grice, J. Intradermal proximal field block: An innovative anesthetic technique for levonorgestrel implant removal. Obstet. Gynecol. 1998, 91, 294–297. [Google Scholar] [CrossRef]
- Benagiano, G.; Gabelnick, H.; Farris, M. Contraceptive devices: Subcutaneous delivery systems. Expert Rev. Med. Devices 2008, 5, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Sivin, I.; Laȧhteenmaȧki, P.; Ranta, S.; Darney, P.; Klaisle, C.; Wan, L.; Mishell, D.R., Jr.; Lacarra, M.; Viegas, O.A.; Bilhareus, P.; et al. Levonorgestrel concentrations during use of levonorgestrel rod (LNG ROD) implants. Contraception 1997, 55, 81–85. [Google Scholar] [CrossRef]
- Steiner, M.J.; Lopez, L.M.; Grimes, D.A.; Cheng, L.; Shelton, J.; Trussell, J.; Farley, T.M.; Dorflinger, L. Sino-implant (II)—A levonorgestrel-releasing two-rod implant: Systematic review of the randomized controlled trials. Contraception 2010, 81, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Sivin, I.; Campodonico, I.; Kiriwat, O.; Holma, P.; Diaz, S.; Wan, L.; Biswas, A.; Viegas, O.; el din Abdalla, K.; Anant, M.P.; et al. The performance of levonorgestrel rod and Norplant contraceptive implants: A 5 year randomized study. Hum. Reprod. 1998, 13, 3371–3378. [Google Scholar] [CrossRef] [Green Version]
- French, R.; Van Vliet, H.; Cowan, F.; Mansour, D.; Morris, S.; Hughes, D.; Robinson, A.; Proctor, T.; Summerbell, C.; Logan, S.; et al. Hormonally impregnated intrauterine systems (IUSs) versus other forms of reversible contraceptives as effective methods of preventing pregnancy. Cochrane Database Syst. Rev. 2004, 3, CD001776. [Google Scholar]
- Hidalgo, M.M.; Lisondo, C.; Juliato, C.T.; Espejo-Arce, X.; Monteiro, I.; Bahamondes, L. Ovarian cysts in users of Implanon and Jadelle subdermal contraceptive implants. Contraception 2006, 73, 532–536. [Google Scholar] [CrossRef]
- Buckshee, K.; Chatterjee, P.; Dhall, G.I.; Hazra, M.N.; Kodkany, B.S.; Lalitha, K.; Logambal, A.; Manchanda, P.; Nanda, U.K.; RaiChoudhury, G. Return of fertility following discontinuation of Norplant-II subdermal implants. ICMR Task Force on Hormonal Contraception. Contraception 1995, 51, 237–242. [Google Scholar] [CrossRef]
- Moray, K.V.; Chaurasia, H.; Sachin, O.; Joshi, B. A systematic review on clinical effectiveness, side-effect profile and meta-analysis on continuation rate of etonogestrel contraceptive implant. Reprod. Health 2021, 18, 4. [Google Scholar] [CrossRef]
- Mansour, D.; Mommers, E.; Teede, H.; Sollie-Eriksen, B.; Graesslin, O.; Ahrendt, H.J.; Gemzell-Danielsson, K. Clinician satisfaction and insertion characteristics of a new applicator to insert radiopaque Implanon: An open-label, noncontrolled, multicenter trial. Contraception 2010, 82, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Wenzl, R. Pharmacokinetics of Implanon. An integrated analysis. Contraception 1998, 58, 85–90. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.S.; Kim, J.S.; Kim, S.; Cho, S. Experiences of localization and removal of non-palpable subdermal contraceptive implants with ultrasound. Obstet. Gynecol. Sci. 2019, 62, 166–172. [Google Scholar] [CrossRef]
- Carlos-Alves, M.; Gomes, M.; Abreu, R.; Pinheiro, P. Lung migration of contraceptive Implanon NXT. BMJ Case Rep. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Bennink, H.J. The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant. Eur. J. Contracept. Reprod. Health Care 2000, 5, 12–20. [Google Scholar]
- Fischer, M.A. Implanon: A new contraceptive implant. J. Obstet. Gynecol. Neonatal. Nurs. 2008, 37, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, H.B.; Makarainen, L. The pharmacodynamics and efficacy of Implanon. An overview of the data. Contraception 1998, 58, 91–97. [Google Scholar] [CrossRef]
- Croxatto, H.B.; Urbancsek, J.; Massai, R.; Coelingh Bennink, H.; van Beek, A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum. Reprod. 1999, 14, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Croxatto, H.B. Clinical profile of Implanon: A single-rod etonogestrel contraceptive implant. Eur. J. Contracept. Reprod. Health Care 2000, 2, 21–28. [Google Scholar]
- Edwards, J.E.; Moore, A. Implanon. A review of clinical studies. Br. J. Fam. Plann. 1999, 24, 3–16. [Google Scholar]
- Flores, J.B.; Balderas, M.L.; Bonilla, M.C.; Vázquez-Estrada, L. Clinical experience and acceptability of the etonogestrel subdermal contraceptive implant. Int. J. Gynaecol. Obstet. 2005, 90, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Holman, R.; Lindsay, E. Migration of Implanon: Two case reports. J. Fam. Plann. Reprod. Health Care 2005, 31, 71–72. [Google Scholar] [CrossRef] [Green Version]
- Harrison-Woolrych, M.; Hill, R. Unintended pregnancies with the etonogestrel implant (Implanon): A case series from postmarketing experience in Australia. Contraception 2005, 71, 306–308. [Google Scholar] [CrossRef]
- Power, J.; French, R.; Cowan, F. Subdermal implantable contraceptives versus other forms of reversible contraceptives or other implants as effective methods of preventing pregnancy. Cochrane Database Syst. Rev. 2007, 3, CD001326. [Google Scholar] [CrossRef]
- Graesslin, O.; Korver, T. The contraceptive efficacy of Implanon: A review of clinical trials and marketing experience. Eur. J. Contracept. Reprod. Health Care 2008, 13, 4–12. [Google Scholar] [CrossRef]
- Bhatia, P.; Nangia, S.; Aggarwal, S.; Tewari, C. Implanon: Subdermal single rod contraceptive implant. J. Obstet. Gynaecol. India 2011, 61, 422–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patni, S.; Ebden, P.; Kevelighan, E.; Bibby, J. Ectopic pregnancy with Implanon. J. Fam. Plann. Reprod. Health Care 2006, 32, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olowu, O.; Karunaratne, J.; Odejinmi, F. Ectopic pregnancy with Implanon® as a method of contraception in a woman with a previous ectopic pregnancy—Case report. Eur. J. Contracept. Reprod. Health Care 2011, 16, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Bouquier, J.; Fulda, V.; Bats, A.S.; Lécuru, F.; Huchon, C. A life-threatening ectopic pregnancy with etonogestrel implant. Contraception 2012, 85, 215–217. [Google Scholar] [CrossRef]
- Adams, K.; Beal, M.W. Implanon: A review of the literature with recommendations for clinical management. J. Midwifery Womens Health 2009, 54, 142–149. [Google Scholar] [CrossRef]
- Mansour, D.; Fraser, I.S.; Edelman, A.; Vieira, C.S.; Kaunitz, A.M.; Korver, T.; Pong, A.; Lin, J.; Shah, A.K.; Fox, M.; et al. Can initial vaginal bleeding patterns in etonogestrel implant users predict subsequent bleeding in the first 2 years of use? Contraception 2019, 100, 264–268. [Google Scholar] [CrossRef]
- Funk, S.; Miller, M.M.; Mishell, D.R., Jr.; Archer, D.F.; Poindexter, A.; Schmidt, J.; Zampaglione, E. Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception 2005, 71, 319–326. [Google Scholar] [CrossRef]
- Blumenthal, P.D.; Gemzell-Danielsson, K.; Marintcheva-Petrova, M. Tolerability and clinical safety of Implanon. Eur. J. Contracept. Reprod. Health Care 2008, 13, 29–36. [Google Scholar] [CrossRef]
- Aisien, A.O.; Enosolease, M.E. Safety, efficacy and acceptability of implanon a single rod implantable contraceptive (etonogestrel) in University of Benin Teaching Hospital. Niger. J. Clin. Pract. 2010, 13, 331–335. [Google Scholar]
- Modesto, W.; Bahamondes, M.V.; Bahamondes, L. A randomized clinical trial of the effect of intensive versus non-intensive counselling on discontinuation rates due to bleeding disturbances of three long-acting reversible contraceptives. Hum. Reprod 2014, 29, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nageso, A.; Gebretsadik, A. Discontinuation rate of Implanon and its associated factors among women who ever used Implanon in Dale District, Southern Ethiopia. BMC Womens Health 2018, 18, 189. [Google Scholar] [CrossRef]
- Peterson, A.M.; Brown, A.; Savage, A.; Dempsey, A. Prevalence of early discontinuation and associated factors among a retrospective cohort of etonogestrel contraceptive implant users. Eur. J. Contracept. Reprod. Health Care 2019, 24, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Mesha, M.; Alemayehu, A.; Daka, D. Prevalence and factors associated with early discontinuation rate of Implanon utilization among women who ever used Implanon in Kucha District Gamo Gofa Zone, Southern Ethiopia. BMC Womens Health 2020, 20, 239. [Google Scholar]
- Weisberg, E.; Hickey, M.; Palmer, D.; O’Connor, V.; Salamonsen, L.A.; Findlay, J.K.; Fraser, I.S. A pilot study to assess the effect of three short-term treatments on frequent and/or prolonged bleeding compared to placebo in women using Implanon. Hum. Reprod. 2006, 21, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisberg, E.; Hickey, M.; Palmer, D.; O’Connor, V.; Salamonsen, L.A.; Findlay, J.K.; Fraser, I.S. A randomized controlled trial of treatment options for troublesome uterine bleeding in Implanon users. Hum. Reprod 2009, 24, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Lazorwitz, A.; Aquilante, C.L.; Dindinger, E.; Harrison, M.; Sheeder, J.; Teal, S. Relationship Between Etonogestrel Concentrations and Bleeding Patterns in Contraceptive Implant Users. Obstet. Gynecol. 2019, 134, 807–813. [Google Scholar] [CrossRef]
- Upawi, S.N.; Ahmad, M.F.; Abu, M.A.; Ahmad, S. Management of bleeding irregularities among etonogestrel implant users: Is combined oral contraceptives pills or nonsteroidal anti-inflammatory drugs the better option? J. Obstet. Gynaecol. Res. 2020, 46, 479–484. [Google Scholar] [CrossRef]
- Merki-Feld, G.S.; Imthurn, B.; Seifert, B. Effects of the progestogen-only contraceptive implant on cardiovascular risk factors. Clin. Endocrinol. 2008, 68, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Merki-Feld, G.S.; Rosselli, M.; Imthurn, B.; Spanaus, K. No effect of Implanon® on inflammatory cardiovascular parameters. Gynecol. Endocrinol. 2011, 27, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Shoupe, D. Effects of desogestrel on carbohydrate metabolism. Am. J. Obstet. Gynecol. 1993, 168, 1041–1047. [Google Scholar] [CrossRef]
- Biswas, A.; Viegas, O.A.; Coeling Bennink, H.J.; Korver, T.; Ratnam, S.S. Implanon contraceptive implants: Effects on carbohydrate metabolism. Contraception 2001, 63, 137–141. [Google Scholar] [CrossRef]
- Oderich, C.L.; Wender, M.C.; Lubianca, J.N.; Santos, L.M.; de Mello, G.C. Impact of etonogestrel-releasing implant and copper intrauterine device on carbohydrate metabolism: A comparative study. Contraception 2012, 85, 173–176. [Google Scholar] [CrossRef]
- Villas-Boas, J.; Vilodre, L.C.; Malerba, H.; Pontremoli Salcedo, M.; Foresti Jiménez, M.; El Beitune, P. Metabolic safety of the etonogestrel contraceptive implant in healthy women over a 3-year period. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 202, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Merki-Feld, G.S.; Imthurn, B.; Seifert, B. Effects of the progestagen-only contraceptive implant Implanon on transforming growth factor beta1 and endothelin-1. Horm. Metab. Res. 2008, 40, 692–696. [Google Scholar] [CrossRef]
- Egberg, N.; van Beek, A.; Gunnervik, C.; Hulkko, S.; Hirvonen, E.; Larsson-Cohn, U.; Bennink, H.C. Effects on the hemostatic system and liver function in relation to Implanon and Norplant. A prospective randomized clinical trial. Contraception 1998, 58, 93–98. [Google Scholar] [CrossRef]
- Nasr, A.; Nafeh, H.M. Effect of etonogestrel contraceptive implant (Implanon) on portal blood flow and liver functions. Contraception 2009, 79, 236–239. [Google Scholar] [CrossRef]
- Pongsatha, S.; Ekmahachai, M.; Suntornlimsiri, N.; Morakote, N.; Chaovisitsaree, S. Bone mineral density in women using the subdermal contraceptive implant Implanon for at least 2 years. Int. J. Gynaecol. Obstet. 2010, 109, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Affandi, B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998, 58, 99S–107S. [Google Scholar] [CrossRef]
- Mäkäräinen, L.; van Beek, A.; Tuomivaara, L.; Asplund, B.; Coelingh Bennink, H. Ovarian function during the use of a single contraceptive implant: Implanon compared with Norplant. Fertil. Steril. 1998, 69, 714–721. [Google Scholar] [CrossRef]
- Zheng, S.R.; Zheng, H.M.; Qian, S.Z.; Sang, G.W.; Kaper, R.F. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon®) and a six-capsule (Norplant®) hormonal contraceptive implant. Contraception 1999, 60, 1–8. [Google Scholar] [CrossRef]
- Meirik, O.; Brache, V.; Orawan, K.; Habib, N.A.; Schmidt, J.; Ortayli, N.; Culwell, K.; Jackson, E.; Ali, M. WHO Study Group on Contraceptive Implants for Women. A multicenter randomized clinical trial of one-rod etonogestrel and two-rod levonorgestrel contraceptive implants with nonrandomized copper-IUD controls: Methodology and insertion data. Contraception 2013, 87, 113–120. [Google Scholar] [CrossRef]
- Okunola, T.O.; Bola-Oyebamiji, S.B.; Sowemimo, O. Comparison of weight gain between levonorgestrel and etonogestrel implants after 12 months of insertion. Int. J. Gynaecol. Obstet. 2019, 147, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Berenson, A.B.; Tan, A.; Hirth, J.M. Complications and continuation rates associated with 2 types of long-acting contraception. Am. J. Obstet. Gynecol. 2015, 212, 761.e1–761.e8. [Google Scholar] [CrossRef] [Green Version]
- 2020 Canadian Agency for Drugs and Technologies in Health. Cadth Common Drug Review. In Clinical Review Report for Etonogestrel (Nexplanon); Merck Canada Inc.: Kirkland, QC, Canada, 2020. [Google Scholar]
- Medical Eligibility Criteria for Implants|Family Planning. Available online: https://www.fphandbook.org/medical-eligibility-criteria-implants (accessed on 8 November 2020).
- Díaz, S.; Pavez, M.; Moo-Young, A.J.; Bardin, C.W.; Croxatto, H.B. Clinical trial with 3-keto-desogestrel subdermal implants. Contraception 1991, 44, 393–408. [Google Scholar] [CrossRef]
- Barlow-Evans, R.; Jaffer, K.; Balogun, M. Migration of a Nexplanon contraceptive implant to the pulmonary artery. BMJ Case Rep. 2017, 2017, bcr2017219259. [Google Scholar] [CrossRef] [PubMed]
- Mommers, E.; Blum, G.F.; Gent, T.G.; Peters, K.P.; Sørdal, T.S.; Marintcheva-Petrova, M. Nexplanon, a radiopaque etonogestrel implant in combination with a next-generation applicator: 3-year results of a noncomparative multicenter trial. Am. J. Obstet. Gynecol. 2012, 207, 388.e1–388.e6. [Google Scholar] [CrossRef] [PubMed]
- Cooling, H.; Pauli, H. Full-term pregnancy with Implanon in situ. J. Fam. Plann. Reprod. Health Care 2006, 32, 204. [Google Scholar] [CrossRef] [Green Version]
- UK Medical Eligibility Criteria for Contraceptive Use (UKMEC 2016); Faculty of Sexual & Reproductive Health Care: London, UK, 2016; Available online: https://www.fsrh.org/standards-and-guidance/external/ukmec-2016-digital-version (accessed on 1 March 2021).
- Mansour, D.; Bahamondes, L.; Critchley, H.; Darney, P.; Fraser, I.S. The management of unacceptable bleeding patterns in etonogestrel-releasing contraceptive implant users. Contraception 2011, 83, 202–210. [Google Scholar] [CrossRef] [PubMed]
- López Del Cerro, E.; Serrano Diana, C.; Castillo Cañadas, A.M.; González Mirasol, E.; García Santos, F.; Gómez García, M.T.; González de Merlo, G. Influence of age on tolerability, safety and effectiveness of subdermal contraceptive implants. J. Obstet. Gynaecol. 2018, 38, 979–984. [Google Scholar] [CrossRef]
- Casey, P.M.; Long, M.E.; Marnach, M.L.; Fleming-Harvey, J.; Drozdowicz, L.B.; Weaver, A.L. Association of body mass index with removal of etonogestrel subdermal implant. Contraception 2013, 87, 370–374. [Google Scholar] [CrossRef]
- Di Carlo, C.; Guida, M.; De Rosa, N.; Sansone, A.; Gargano, V.; Cagnacci, A.; Nappi, C. Bleeding profile in users of an etonogestrel sub-dermal implant: Effects of anthropometric variables. An observational uncontrolled preliminary study in Italian population. Gynecol. Endocrinol. 2015, 31, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.Y.; McNicholas, C.; Creinin, M.D. Combined oral contraceptive treatment for bleeding complaints with the etonogestrel contraceptive implant: A randomised controlled trial. Eur. J. Contracept. Reprod Health Care 2016, 21, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Edelman, A.B.; Kaneshiro, B.; Simmons, K.B.; Hauschildt, J.L.; Bond, K.; Boniface, E.R.; Jensen, J.T. Treatment of Unfavorable Bleeding Patterns in Contraceptive Implant Users: A Randomized Controlled Trial. Obstet. Gynecol. 2020, 136, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Guazzelli, C.A.F.; de Queiroz, F.T.; Barbieri, M.; Barreiros, F.A.; Torloni, M.R.; Araujo, F.F. Metabolic effects of contraceptive implants in adolescents. Contraception 2011, 84, 409–412. [Google Scholar] [CrossRef]
- Guida, M.; Farris, M.; Aquino, C.I.; Rosato, E.; Cipullo, L.M.A.; Bastianelli, C. Nexplanon Subdermal Implant: Assessment of Sexual Profile, Metabolism, and Bleeding in a Cohort of Italian Women. BioMed Res. Int. 2019, 2019, 3726957. [Google Scholar] [CrossRef] [Green Version]
- Modesto, W.; Dal Ava, N.; Monteiro, I.; Bahamondes, L. Body composition and bone mineral density in users of the etonogestrel-releasing contraceptive implant. Arch. Gynecol. Obstet. 2015, 292, 1387–1391. [Google Scholar] [CrossRef]
- Vickery, Z.; Madden, T.; Zhao, Q.; Secura, G.M.; Allsworth, J.E.; Peipert, J.F. Weight change at 12 months in users of three progestin-only contraceptive methods. Contraception 2013, 88, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Lidegaard, Ø.; Løkkegaard, E.; Jensen, A.; Skovlund, C.W.; Keiding, N. Thrombotic stroke and myocardial infarction with hormonal contraception. N. Engl. J. Med. 2012, 366, 2257–2266. [Google Scholar] [CrossRef] [Green Version]
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary hormonal contraception and the risk of breast cancer. N. Engl. J. Med. 2017, 377, 2228–2239. [Google Scholar] [CrossRef]
- Iversen, L.; Fielding, S.; Lidegaard, Ø.; Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: Prospective, nationwide cohort study. BMJ 2018, 26, k3609. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.; Do Minh, T.; Lange, J.A.; Koro, C.; Fox, M.; Heinemann, K. Real world data on Nexplanon® procedure-related events: Final results from the Nexplanon Observational Risk Assessment study (NORA). Contraception 2019, 100, 31–36. [Google Scholar] [CrossRef]
- Gao, G.T.; Binder, W. Embolization of a contraceptive implant into the pulmonary vasculature in an adolescent female. Am. J. Emerg. Med. 2018, 36, 1122.e1–1122.e2. [Google Scholar] [CrossRef]
- Chaudry, F. Adverse Reaction to Nexplanon (R). J. Fam. Plan. Reprod. Health Care 2013, 39, 231–232. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M. Allergy to Nexplanon®. J. Fam. Plan. Reprod. Health Care 2012, 38, 272. [Google Scholar] [CrossRef] [Green Version]
- Pedroso, C.; Martins, I.; Palma, F.; Machado, A.I. Implant site Nexplanon reaction? BMJ Case Rep. 2015, 2015, bcr2014206256. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.M.; Caggiano, N.M.; Giladi, A.M.; Iorio, M.L. Median Nerve Injury after Removal of Subdermal Implantable Contraceptive. Hand 2018, 13, NP6–NP9. [Google Scholar] [CrossRef]
- Saeed, A.; Narayan, N.; Pandya, A. Contraceptive Implant-Related Acute Ulnar Neuropathy: Prompt Diagnosis, Early Referral, and Management Are Key. Eplasty 2018, 18, e28. [Google Scholar]
- Simon, C.; Maurier, A.; Gaboriau, L.; Vrignaud, L.; Dayani, P.; Vaillant, T.; Andrée Bos-Thompson, M.; Jonville-Bera, A.P. Incidence and characteristics of intravascular pulmonary migration of etonogestrel implants: A French nationwide study. Contraception 2020, 102, 186–189. [Google Scholar] [CrossRef]
- Diego, D.; Tappy, E.; Carugno, J. Axillary migration of Nexplanon®: Case report. Contraception 2017, 95, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Park, J.U.; Bae, H.S.; Lee, S.M.; Bae, J.; Park, J.W. Removal of a subdermal contraceptive implant (Implanon NXT) that migrated to the axilla by C-arm guidance: A case report and review of the literature. Medicine 2017, 96, e8627. [Google Scholar] [CrossRef]
- Thomas, P.A.; Di Stefano, D.; Couteau, C.; D’Journo, X.B. Contraceptive Implant Embolism Into the Pulmonary Artery: Thoracoscopic Retrieval. Ann. Thorac. Surg. 2017, 103, e271–e272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kew, E.P.; Senanayake, E.; Djearaman, M.; Bishay, E. Migration of contraceptive implant into the left pulmonary arterial system. Asian Cardiovasc. Thorac. Ann. 2017, 25, 537–539. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, H.Y.; Lee, S.S.; Cho, S. Migration of a contraceptive subdermal device into the lung. Obstet. Gynecol. Sci. 2017, 60, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Ohannessian, A.; Levy, A.; Jaillant, N.; Tanguy Le Gac, Y.; D’Journo, X.; Vidal, V.; Agostini, A. A French survey of contraceptive implant migration to the pulmonary artery. Contraception 2019, 100, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Casado-Espada, N.M.; de Alarcón, R.; de la Iglesia-Larrad, J.I.; Bote-Bonaechea, B.; Montejo, Á.L. Hormonal Contraceptives, Female Sexual Dysfunction, and Managing Strategies: A Review. J. Clin. Med. 2019, 8, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carlo, C.; Sansone, A.; De Rosa, N.; Gargano, V.; Tommaselli, G.A.; Nappi, C.; Bifulco, G. Impact of an implantable steroid contraceptive (etonogestrel-releasing implant) on quality of life and sexual function: A preliminary study. Gynecol. Endocrinol. 2014, 30, 53–56. [Google Scholar] [CrossRef]
- Moreira, I.F.A.; Bianchini, M.P.; Moreira, G.R.C.; Almeida, A.M.; Rezende, B.A. Sexual function and metabolic/hormonal changes in women using long-term hormonal and non-hormonal contraceptives: A pilot study. BMC Womens Health 2020, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Chapa, H.O.; Ramirez, A.; Dawson, D. Etonogestrel contraceptive implant-associated secondary anorgasmia. Contraception 2017, 96, 254–256. [Google Scholar] [CrossRef]
- Lazorwitz, A.; Aquilante, C.L.; Sheeder, J.; Guiahi, M.; Teal, S. Relationship between patient characteristics and serum etonogestrel concentrations in contraceptive implant users. Contraception 2019, 100, 37–41. [Google Scholar] [CrossRef]
- Morrell, K.M.; Cremers, S.; Westhoff, C.L.; Davis, A.R. Relationship between etonogestrel level and BMI in women using the contraceptive implant for more than 1 year. Contraception 2016, 93, 263–265. [Google Scholar] [CrossRef]
- Mornar, S.; Chan, L.N.; Mistretta, S.; Neustadt, A.; Martins, S.; Gilliam, M. Pharmacokinetics of the etonogestrel contraceptive implant in obese women. Am. J. Obstet. Gynecol. 2012, 207, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Lazorwitz, A.; Sheeder, J.; Teal, S. An exploratory study on the association of lifestyle factors with serum etonogestrel concentrations among contraceptive implant users. Eur. J. Contracept. Reprod. Health Care 2021, 1–7. [Google Scholar] [CrossRef]
- Xu, H.; Wade, J.A.; Peipert, J.F.; Zhao, Q.; Madden, T.; Secura, G.M. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstet. Gynecol. 2012, 120, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, L.M.; Bernholc, A.; Chen, M.; Grey, T.W.; Otterness, C.; Westhoff, C.; Edelman, A.; Helmerhorst, F.M. Hormonal contraceptives for contraception in overweight or obese women. Cochrane Database Syst. Rev. 2016, 8, CD008452. [Google Scholar] [CrossRef]
- Bender, N.M.; Segall-Gutierrez, P.; Najera, S.O.; Stanczyk, F.Z.; Montoro, M.; Mishell, D.R., Jr. Effects of progestin-only long-acting contraception on metabolic markers in obese women. Contraception 2013, 88, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.M.; Grimes, D.A.; Schulz, K.F. Steroidal contraceptives: Effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- Schnabel, P.; Merki-Feld, G.S.; Malvy, A.; Duijkers, I.; Mommers, E.; van den Heuvel, M.W. Bioequivalence and X-ray visibility of a radiopaque etonogestrel implant versus a non-radiopaque implant: A 3-year, randomized, double-blind study. Clin. Drug Investig. 2012, 32, 413–422. [Google Scholar] [CrossRef]
- Bahamondes, L.; Brache, V.; Meirik, O.; Ali, M.; Habib, N.; Landoulsi, S.; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum. Reprod. 2015, 30, 2527–2538. [Google Scholar] [CrossRef]
- Bahamondes, L.; Brache, V.; Ali, M.; Habib, N.; WHO Study Group on Contraceptive Implants for Women. A multicenter randomized clinical trial of etonogestrel and levonorgestrel contraceptive implants with nonrandomized copper intrauterine device controls: Effect on weight variations up to 3 years after placement. Contraception 2018, 98, 181–187. [Google Scholar] [CrossRef]
- Ali, M.; Bahamondes, L.; Bent Landoulsi, S. Extended effectiveness of the etonogestrel-releasing contraceptive implant and the 20 mg levonorgestrel-releasing intrauterine system for 2 Years beyond U.S. Food and Drug administration product labeling. Glob. Health Sci. Pract. 2017, 5, 534–539. [Google Scholar] [CrossRef] [Green Version]
- McNicholas, C.; Maddipati, R.; Zhao, Q.; Swor, E.; Peipert, J.F. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet. Gynecol. 2015, 125, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Short, M.; Dallay, D.; Omokanye, S.; Hanisch, J.U.; Inki, P. Acceptability of the levonorgestrel releasing-intrauterine system and etonogestrel implant: One-year results of an observational study. Eur. J. Contracept. Reprod. Health Care 2012, 17, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Short, M.; Dallay, D.; Omokanye, S.; Stauch, K.; Inki, P. Acceptability of long-acting, progestin-only contraception in Europe: A two-year prospective, non-interventional study. Eur. J. Contracept. Reprod. Health Care 2014, 19, 29–38. [Google Scholar] [CrossRef]
- Apter, D.; Briggs, P.; Tuppurainen, M.; Grunert, J.; Lukkari-Lax, E.; Rybowski, S.; Gemzell-Danielsson, K. A 12-month multicenter, randomized study comparing the levonorgestrel intrauterine system with the etonogestrel subdermal implant. Fertil. Steril. 2016, 106, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, J.N.; Turok, D.K.; Gawron, L.M.; Law, A.; Wen, L.; Lynen, R. Two-year continuation of intrauterine devices and contraceptive implants in a mixed-payer setting: A retrospective review. Am. J. Obstet. Gynecol. 2017, 216, 590.e1–590.e8. [Google Scholar] [CrossRef] [Green Version]
- Law, A.; Liao, L.; Lin, J.; Yaldo, A.; Lynen, R. Twelve-month discontinuation rates of levonorgestrel intrauterine system 13.5 mg and subdermal etonogestrel implant in women aged 18–44: A retrospective claims database analysis. Contraception 2018, 98, 120–124. [Google Scholar] [CrossRef]
- Saloranta, T.H.; Gyllenberg, F.K.; But, A.; Gissler, M.; Laine, M.K.; Heikinheimo, O. Free-of-charge long-acting reversible contraception: Two-year discontinuation, its risk factors, and reasons. Am. J. Obstet. Gynecol. 2020, 223, 886.e1–886.e17. [Google Scholar] [CrossRef]
- McNicholas, C.; Swor, E.; Wan, L.; Peipert, J.F. Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration. Am. J. Obstet. Gynecol. 2017, 216, 586.e1–586.e6. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Akin, A.; Bahamondes, L.; Brache, V.; Habib, N.; Landoulsi, S.; Hubacher, D. WHO study group on subdermal contraceptive implants for women. Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: Comparison to levonorgestrel-releasing subdermal implant. Hum. Reprod. 2016, 31, 2491–2498. [Google Scholar] [CrossRef] [Green Version]
- Kiriwat, O.; Patanayindee, A.; Koetsawang, S.; Korver, T.; Bennink, H.J. A 4-year pilot study on the efficacy and safety of Implanon, a single-rod hormonal contraceptive implant, in healthy women in Thailand. Eur. J. Contracept. Reprod. Health Care 1998, 3, 85–91. [Google Scholar] [CrossRef]
- Todd, N.; Black, A. Contraception for Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 28–40. [Google Scholar] [CrossRef]
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Work Group. ACOG Committee Opinion No. 735: Adolescents and Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Obstet. Gynecol. 2018, 131, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Curtis, K.M.; Jatlaoui, T.C.; Tepper, N.K.; Zapata, L.B.; Horton, L.G.; Jamieson, D.J.; Whiteman, M.K. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recomm. Rep. 2016, 65, 1–66. [Google Scholar]
- Committee on Adolescent Health Care. ACOG Committee Opinion No 699: Adolescent Pregnancy, Contraception, and Sexual Activity. Obstet. Gynecol. 2017, 129, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, G.; Crowther, C.; Simms, J. Contraceptive care for Canadian youth. Paediatr. Child. Health 2018, 23, 271–277. [Google Scholar] [CrossRef]
- Black, A.; Guilbert, E.; Costescu, D.; Dunn, S.; Fisher, W.; Kives, S.; Mirosh, M.; Norman, W.; Pymar, H.; Reid, R.; et al. Canadian Contraception Consensus (Part 3 of 4): Chapter 7—Intrauterine Contraception. methods to prevent pregnancy and on the promotion of healthy sexuality. J. Obstet. Gynaecol. Can. 2016, 38, 182–222. [Google Scholar] [CrossRef]
- World Health Organization. Reproductive Health and Research. In Medical Eligibility Criteria for Contraceptive Use; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Berlan, E.; Mizraji, K.; Bonny, A.E. Twelve-month discontinuation of etonogestrel implant in an outpatient pediatric setting. Contraception 2016, 94, 81–86. [Google Scholar] [CrossRef]
- Obijuru, L.; Bumpus, S.; Auinger, P.; Baldwin, C.D. Etonogestrel Implants in Adolescents: Experience, Satisfaction, and Continuation. J. Adolesc. Health 2016, 58, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Diedrich, J.T.; Klein, D.A.; Peipert, J.F. Long-acting reversible contraception in adolescents: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 364.e1–364.e12. [Google Scholar] [CrossRef] [Green Version]
- Buyers, E.; Sass, A.E.; Severn, C.D.; Pyle, L.; Cree-Green, M. Twelve-month Continuation of the Etonogestrel Implant in Adolescents with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2021, 34, 33–39. [Google Scholar] [CrossRef]
- Romano, M.E.; Braun-Courville, D.K. Assessing Weight Status in Adolescent and Young Adult Users of the Etonogestrel Contraceptive Implant. J. Pediatr. Adolesc. Gynecol. 2019, 32, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Amies Oelschlager, A.M.; Micks, E.A.; Debiec, K.E.; Nizamic, T.; Mantrala, M.D.; Prager, S.W. Long acting reversible contraception in adolescents with cardiovascular conditions. J. Pediatr. Adolesc. Gynecol. 2014, 27, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.; Merino, P.M.; Giraudo, F.; Codner, E. Long-acting contraception in adolescents and young women with type 1 and type 2 diabetes. Pediatr. Diabetes 2020, 21, 1074–1082. [Google Scholar] [CrossRef]
- Berlan, E.D.; Richards, M.J.; Vieira, C.S.; Creinin, M.D.; Kaunitz, A.M.; Fraser, I.S.; Edelman, A.; Mansour, D. Best Practices for Counseling Adolescents about the Etonogestrel Implant. J. Pediatr. Adolesc. Gynecol. 2020, 3, 448–454. [Google Scholar] [CrossRef]
- Lazorwitz, A.; Coleman-Minahan, K.; Teal, S.B.; Guiahi, M. Ongoing Etonogestrel Contraceptive Implant Use Throughout Pregnancy. J. Adolesc. Health 2018, 63, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, J.E.; Upadhyay, U.D.; Sokoloff, A.; Harper, C.C.; Diedrich, J.T.; Drey, E.A. Choice of the levonorgestrel intrauterine device, etonogestrel implant or depot medroxyprogesterone acetate for contraception after aspiration abortion. Contraception 2015, 92, 553–559. [Google Scholar] [CrossRef]
- Barbieri, M.M.; Juliato, C.R.T.; Bahamondes, L.; Surita, F.G. ENG-releasing subdermal implants in postpartum teenagers—An open-label trial study protocol. Reprod. Health 2020, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Mwalwanda, C.S.; Black, K.I. Immediate post-partum initiation of intrauterine contraception and implants: A review of the safety and guidelines for use. Aust. N. Z. J. Obstet. Gynaecol. 2013, 53, 331–337. [Google Scholar] [CrossRef]
- Sothornwit, J.; Werawatakul, Y.; Kaewrudee, S.; Lumbiganon, P.; Laopaiboon, M. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database Syst. Rev. 2017, 4, CD011913. [Google Scholar] [CrossRef]
- Gurtcheff, S.E.; Turok, D.K.; Stoddard, G.; Murphy, P.A.; Gibson, M.; Jones, K.P. Lactogenesis after early postpartum use of the contraceptive implant: A randomized controlled trial. Obstet. Gynecol. 2011, 117, 1114–1121. [Google Scholar] [CrossRef]
- Wilson, S.; Tennant, C.; Sammel, M.D.; Schreiber, C. Immediate postpartum etonogestrel implant: A contraception option with long-term continuation. Contraception 2014, 90, 259–264. [Google Scholar] [CrossRef]
- Vieira, C.S.; de Nadai, M.N.; de Melo Pereira do Carmo, L.S.; Braga, G.C.; Infante, B.F.; Stifani, B.M.; Ferriani, R.A.; Quintana, S.M. Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, weight change, 12-month continuation and satisfaction rates: A randomized controlled trial. Contraception 2019, 100, 258–263. [Google Scholar] [CrossRef]
- Ireland, L.D.; Goyal, V.; Raker, C.A.; Murray, A.; Allen, R.H. The effect of immediate postpartum compared to delayed postpartum and interval etonogestrel contraceptive implant insertion on removal rates for bleeding. Contraception 2014, 90, 253–258. [Google Scholar] [CrossRef]
- Bryant, A.G.; Bauer, A.E.; Stuart, G.S.; Levi, E.E.; Zerden, M.L.; Danvers, A.; Garrett, J.M. Etonogestrel-releasing contraceptive implant for postpartum adolescents: A randomized controlled trial. J. Pediatr. Adolesc. Gynecol. 2017, 30, 389–394. [Google Scholar] [CrossRef]
- Barbieri, M.M.; Herculano, T.; Dantas Silva, A.; Bahamondes, L.; Juliato, C.R.T.; Surita, F.G. Acceptability of ENG-releasing subdermal implants before discharge in Brazilian young women during the COVID-19 pandemic. Int. J. Gynaecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Piva, I.; Brusca, F.; Tassinati, F.; Bonipozzi, S.; Palano, A.; Sassi, M.T.; Bonaccorsi, G.; Morano, D.; Martinello, R.; Scutiero, G.; et al. Post-abortion long-acting reversible contraception in a sample of Italian women: Intrauterine device versus subdermal implant. Gynecol. Endocrinol. 2019, 35, 427–433. [Google Scholar] [CrossRef]
- Griffin, L.; Hammond, C.; Liu, D.; Rademaker, A.W.; Kiley, J. Postpartum weight loss in overweight and obese women using the etonogestrel subdermal implant: A pilot study. Contraception 2017, 95, 564–570. [Google Scholar] [CrossRef]
- Dobromilsky, K.C.; Allen, P.L.; Raymond, S.H.; Maindiratta, B. A prospective cohort study of early postpartum etonogestrel implant (Implanon®) use and its effect on duration of lochia. J. Fam. Plann. Reprod. Health Care 2016, 42, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Brito, M.B.; Ferriani, R.A.; Quintana, S.M.; Yazlle, M.E.; Silva de Sá, M.F.; Vieira, C.S. Safety of the etonogestrel-releasing implant during the immediate postpartum period: A pilot study. Contraception 2009, 80, 519–526. [Google Scholar] [CrossRef]
- Brito, M.B.; Ferriani, R.A.; Meijers, J.C.; Garcia, A.A.; Quintana, S.M.; Silva de Sá, M.F.; Vieira, C.S. Effects of the etonogestrel-releasing contraceptive implant inserted immediately postpartum on maternal hemostasis: A randomized controlled trial. Thromb. Res. 2012, 130, 355–360. [Google Scholar] [CrossRef]
- Floyd, J.L.; Beasley, A.D.; Swaim, L.S.; Turrentine, M.A.; Nijjar, J.B. Association of Immediate Postpartum Etonogestrel Implant Insertion and Venous Thromboembolism. Obstet. Gynecol. 2020, 135, 1275–1280. [Google Scholar] [CrossRef]
- Iltemir Duvan, C.; Onaran, Y.; Aktepe Keskin, E.; Yüce, E.; Yanık, B.; Kafali, H.; Ozturk Turhan, N. Effects of the etonogestrel contraceptive implant (Implanon®) on bone metabolism during lactation: A prospective study. J. Fam. Plann. Reprod. Health Care 2017, 43, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Ti, A.; Curtis, K.M. Postpartum hormonal contraception use and incidence of postpartum depression: A systematic review. Eur. J. Contracept. Reprod. Health Care 2019, 24, 109–116. [Google Scholar] [CrossRef]
- Drake, E.; Grush, K.; Sheeder, J.; Tocce, K. The Association between Immediate Postpartum Etonogestrel Implants and Positive Postpartum Depression Screens in Adolescents and Young Adults. J. Pediatr. Adolesc. Gynecol. 2020, 33, 550–554. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grey, T.W.; Stuebe, A.M.; Chen, M.; Truitt, S.T.; Gallo, M.F. Combined hormonal versus nonhormonal versus progestin-only contraception in lactation. Cochrane Database Syst. Rev. 2015, 3, CD003988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, G.C.; Ferriolli, E.; Quintana, S.M.; Ferriani, R.A.; Pfrimer, K.; Vieira, C.S. Immediate postpartum initiation of etonogestrel-releasing implant: A randomized controlled trial on breastfeeding impact. Contraception 2015, 92, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Bryant, A.G.; Lewis, R.; Muddana, A. Association of Etonogestrel-Releasing Contraceptive Implant with Reduced Weight Gain in an Exclusively Breastfed Infant: Report and Literature Review. Breastfeed. Med. 2016, 11, 203–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmo, L.S.M.P.; Braga, G.C.; Ferriani, R.A.; Quintana, S.M.; Vieira, C.S. Timing of Etonogestrel-Releasing Implants and Growth of Breastfed Infants: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 130, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Taneepanichskul, S.; Reinprayoon, D.; Thaithumyanon, P.; Praisuwanna, P.; Tosukhowong, P.; Dieben, T. Effects of the etonogestrel-releasing implant Implanon and a nonmedicated intrauterine device on the growth of breast-fed infants. Contraception 2006, 73, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Krashin, J.W.; Lemani, C.; Nkambule, J.; Talama, G.; Chinula, L.; Flax, V.L.; Stuebe, A.M.; Tang, J.H. A Comparison of Breastfeeding Exclusivity and Duration Rates between Immediate Postpartum Levonorgestrel Versus Etonogestrel Implant Users: A Prospective Cohort Study. Breastfeed. Med. 2019, 14, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.B.; Garrett, S.M. Postabortion Initiation of Long-Acting Reversible Contraception by Adolescent and Nulliparous Women in New Zealand. J. Adolesc. Health 2016, 58, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Mogeni, R.; Mokua, J.A.; Mwaliko, E.; Tonui, P. Predictors of contraceptive implant uptake in the immediate postpartum period: A cross-sectional study. Eur. J. Contracept. Reprod. Health Care 2019, 24, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Madden, T.; Eisenberg, D.L.; Zhao, Q.; Buckel, C.; Secura, G.M.; Peipert, J.F. Continuation of the etonogestrel implant in women undergoing immediate postabortion placement. Obstet. Gynecol. 2012, 120, 1053–1059. [Google Scholar] [CrossRef]
- Cowett, A.A.; Ali, R.; Cooper, M.A.; Evans, M.; Conzuelo, G.; Cremer, M. Timing of Etonogestrel Implant Insertion After Dilation and Evacuation: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 131, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.; Sonalkar, S.; Borgatta, L. One-year continuation of the etonogestrel contraceptive implant in women with postabortion or interval placement. Contraception 2013, 88, 619–623. [Google Scholar] [CrossRef]
- Roe, A.H.; Fortin, J.; Janiak, E.; Maurer, R.; Goldberg, A.B. Prevalence and predictors of initiation of intrauterine devices and subdermal implants immediately after surgical abortion. Contraception 2019, 100, 89–95. [Google Scholar] [CrossRef]
- Caruso, S.; Vitale, S.G.; Fava, V.; Pasqua, S.D.; Rapisarda, A.M.C.; Cianci, S. Quality of life of women using the etonogestrel long-acting reversible contraceptive implant after abortion for unplanned pregnancy. Eur. J. Contracept. Reprod. Health Care 2020, 25, 251–258. [Google Scholar] [CrossRef]
- Barros Pereira, I.; Carvalho, R.M.; Graça, L.M. Intra-abortion contraception with etonogestrel subdermal implant. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 185, 33–35. [Google Scholar] [CrossRef]
- Hognert, H.; Kopp Kallner, H.; Cameron, S.; Nyrelli, C.; Jawad, I.; Heller, R.; Aronsson, A.; Lindh, I.; Benson, L.; Gemzell-Danielsson, K. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion-a randomized controlled equivalence trial. Hum. Reprod. 2016, 31, 2484–2490. [Google Scholar] [CrossRef]
- Raymond, E.G.; Weaver, M.A.; Tan, Y.L.; Louie, K.S.; Bousiéguez, M.; Lugo-Hernández, E.M.; Aranguré-Peraza, A.G.; Sanhueza, P.; Kaplan, C.; Sonalkar, S.; et al. Effect of Immediate Compared With Delayed Insertion of Etonogestrel Implants on Medical Abortion Efficacy and Repeat Pregnancy: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 127, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Robinson, N.; Wessels, U.; Turner, J.; Geller, S. Progestin-based contraceptive on the same day as medical abortion. Int. J. Gynaecol. Obstet. 2016, 133, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Hansen, M.; Hawkins, J.E.; Lord, J.; Williams, K.; Lohr, P.A.; Hasler, E.; Cameron, S. Long-acting reversible contraception immediately after medical abortion: Systematic review with meta-analyses. Hum. Reprod. Update 2020, 26, 141–160. [Google Scholar] [CrossRef]
- Bahamondes, L.; Valeria Bahamondes, M.; Shulman, L.P. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum. Reprod. Update 2015, 21, 640–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, A.; De Rosa, N.; Giampaolino, P.; Guida, M.; Laganà, A.S.; Di Carlo, C. Effects of etonogestrel implant on quality of life, sexual function, and pelvic pain in women suffering from endometriosis: Results from a multicenter, prospective, observational study. Arch. Gynecol. Obstet. 2018, 298, 731–736. [Google Scholar] [CrossRef]
- Wong, S.; Naresh, A. Etonogestrel Subdermal Implant-Associated Regression of Endometrial Intraepithelial Neoplasia. Obstet. Gynecol. 2019, 133, 780–782. [Google Scholar] [CrossRef]
- Viganò, P.; Parazzini, F.; Somigliana, E.; Vercellini, P. Endometriosis: Epidemiology and aetiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 177–200. [Google Scholar] [CrossRef]
- Koga, K.; Takamura, M.; Fujii, T.; Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil. Steril. 2015, 104, 793–801. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. European Society of Human Reproduction and Embryology. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Benagiano, G.; Guo, S.W.; Bianchi, P.; Puttemans, P.; Gordts, S.; Petraglia, F.; Brosens, I. Pharmacologic treatment of the ovarian endometrioma. Expert Opin. Pharmacother. 2016, 17, 2019–2031. [Google Scholar] [CrossRef]
- Visconti, F.; Di Carlo, C. (Eds.) Endometriosis Pathogenesis, Clinical Impact and Management. In Fronters in Gynecological Endocrinology; ISGE Series; Springer: Cham, Switzerland, 2020; Chapter 7; Volume 9. [Google Scholar]
- Yisa, S.B.; Okenwa, A.A.; Husemeyer, R.P. Treatment of pelvic endometriosis with etonogestrel subdermal implant (Implanon). J. Fam. Plann. Reprod. Health Care 2005, 31, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Walch, K.; Unfried, G.; Huber, J.; Kurz, C.; van Trotsenburg, M.; Pernicka, E.; Wenzl, R. Implanon® versus medroxyprogesterone acetate: Effects on pain scores in patients with symptomatic endometriosis—A pilot study. Contraception 2009, 79, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Margatho, D.; Cursino, K.; Benetti-Pinto, C.L.; Bahamondes, L. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: Randomized clinical trial. Fertil. Steril. 2018, 110, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, X.; Luo, Q.; Wang, C.; Zhu, L.; Huang, L. Effects of Etonogestrel implants on pelvic pain and menstrual flow in women suffering from adenomyosis or endometriosis: Results from a prospective, observational study. Medicine 2021, 100, e24597. [Google Scholar] [CrossRef]
- Ferrero, S.; Scala, C.; Ciccarelli, S.; Vellone, V.G.; Barra, F. Treatment of rectovaginal endometriosis with the etonogestrel-releasing contraceptive implant. Gynecol. Endocrinol. 2020, 36, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Margatho, D.; Carvalho, N.M.; Eloy, L.; Bahamondes, L. Assessment of biomarkers in women with endometriosis-associated pain using the ENG contraceptive implant or the 52 mg LNG-IUS: A non-inferiority randomised clinical trial. Eur. J. Contracept. Reprod. Health Care 2018, 23, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Margatho, D.; Carvalho, N.M.; Bahamondes, L. Endometriosis-associated pain scores and biomarkers in users of the etonogestrel-releasing subdermal implant or the 52-mg levonorgestrel-releasing intrauterine system for up to 24 months. Eur. J. Contracept. Reprod. Health Care 2020, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Mascarenhas, L. Endometrial effects of etonogestrel (Implanon) contraceptive implant. Curr. Opin. Obstet. Gynecol. 2001, 13, 335–341. [Google Scholar] [CrossRef]
- Mascarenhas, L.; van Beek, A.; Bennink, H.C.; Newton, J. A 2-year comparative study of endometrial histology and cervical cytology of contraceptive implant users in Birmingham, UK. Hum. Reprod. 1998, 13, 3057–3060. [Google Scholar] [CrossRef] [Green Version]
- Branum, A.M.; Jones, J. Trends in Long-Acting Reversible Contraception Use among US Women Aged 15–44; Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2015; Volume 188, pp. 1–8. [Google Scholar]
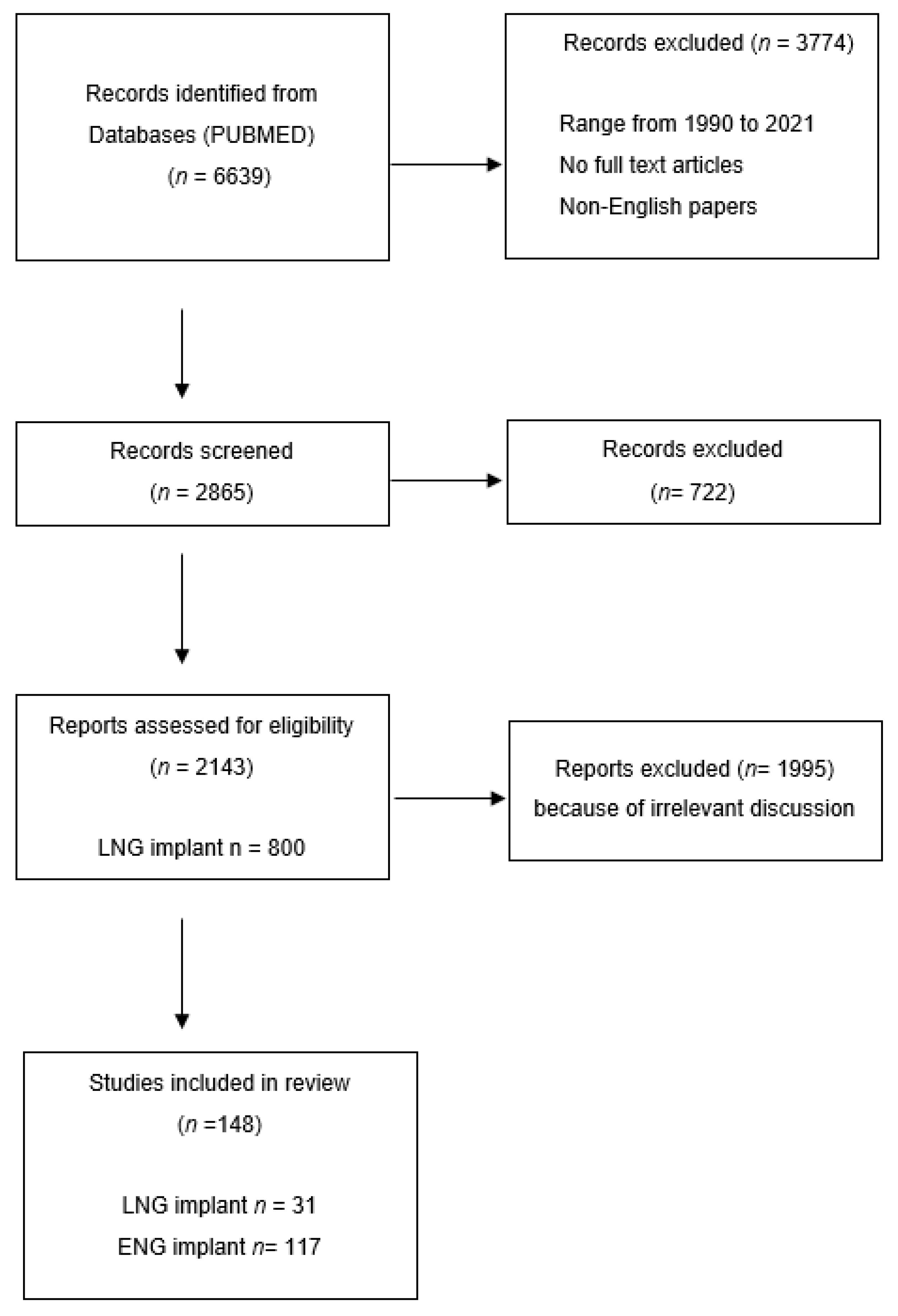
| Subdermal Implants | Norplant | Norplant II (Jadelle) | Implanon | Nexplanon |
|---|---|---|---|---|
| Composition | LNG 6-capsule | LNG-2 silastic rods | ENG-single rod implant | ENG- rod-shaped radio-opaque contraceptive ENG containing barium |
| Dosage | each containing 36 mg LNG | each containing 75 mg LNG | containing 68 mg ENG | containing 68 mg ENG |
| Duration | 5 years | 5 years | 3 years | 3 years |
| Principal medical reason for removal | irregular menstrual bleeding | irregular menstrual bleeding | irregular menstrual bleeding | irregular menstrual bleeding |
| Trackable | NO | NO | NO | YES |
| Mean time taken to remove | 9.59 min | 4.84 min | 2.18 min | <2 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocca, M.L.; Palumbo, A.R.; Visconti, F.; Di Carlo, C. Safety and Benefits of Contraceptives Implants: A Systematic Review. Pharmaceuticals 2021, 14, 548. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060548
Rocca ML, Palumbo AR, Visconti F, Di Carlo C. Safety and Benefits of Contraceptives Implants: A Systematic Review. Pharmaceuticals. 2021; 14(6):548. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060548
Chicago/Turabian StyleRocca, Morena Luigia, Anna Rita Palumbo, Federica Visconti, and Costantino Di Carlo. 2021. "Safety and Benefits of Contraceptives Implants: A Systematic Review" Pharmaceuticals 14, no. 6: 548. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060548






