What the Cardiologist Needs to Consider in the Management of Oncologic Patients with STEMI-Like Syndrome: A Case Report and Literature Review
Abstract
:1. Introduction
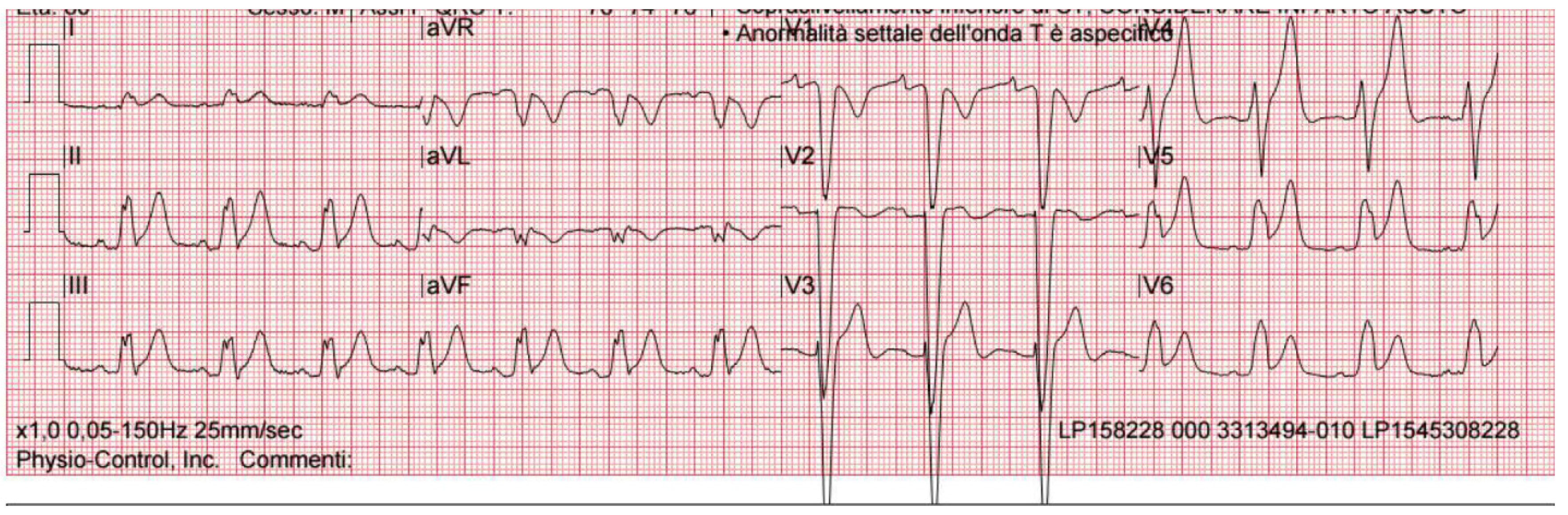
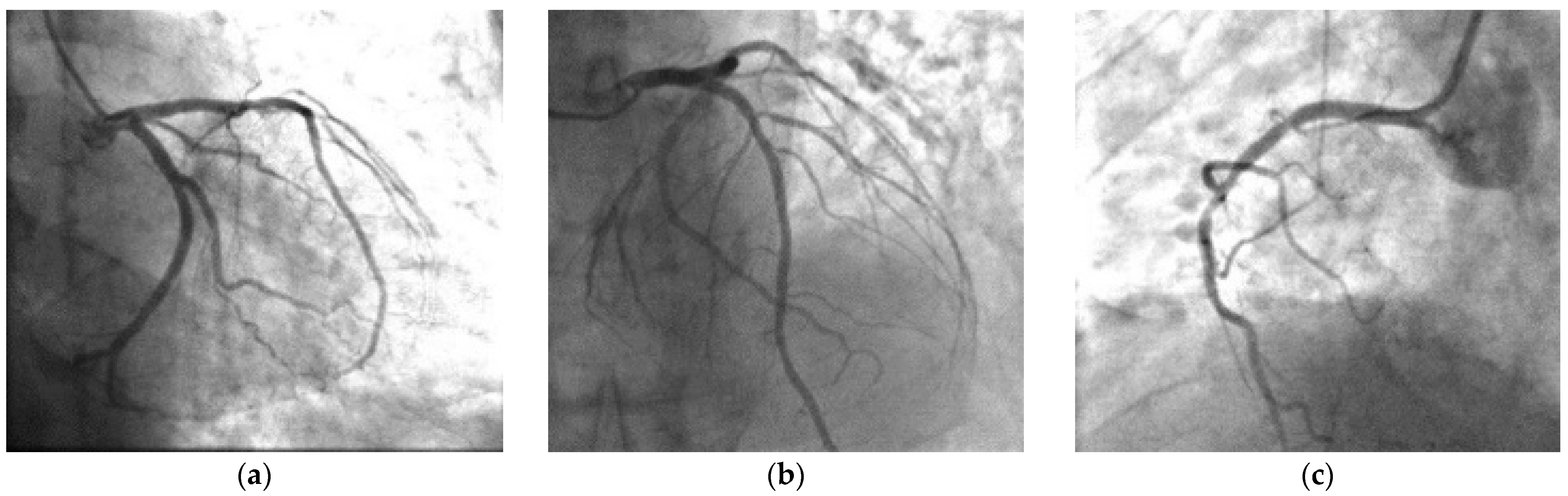
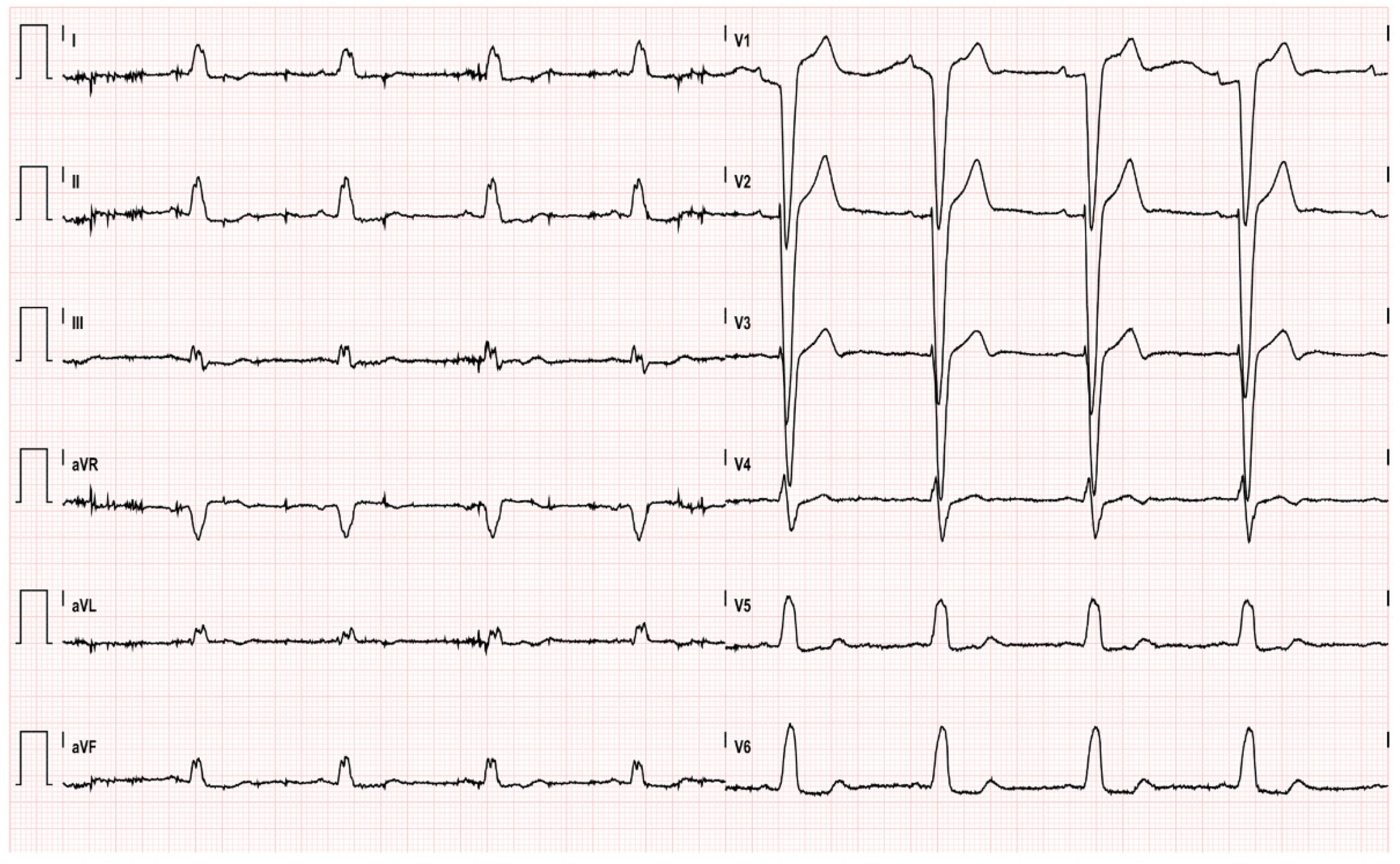
2. Case Presentation
3. Discussion
3.1. Indications for 5-FU
3.2. 5-FU Toxicity and Risk Stratification
3.3. 5-FU Cardiotoxicity from a Clinical Point of View
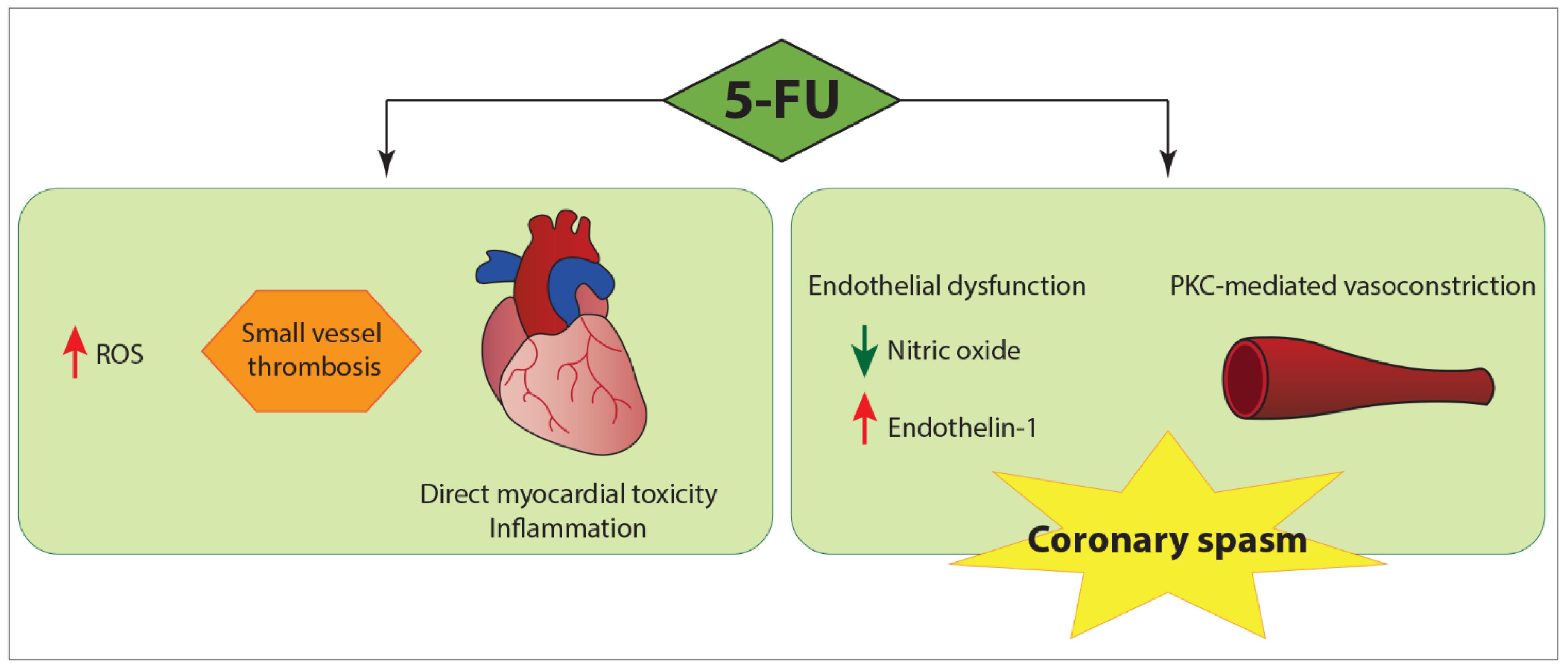
3.4. Underlying Pathogenic Mechanisms
3.5. Management of 5-FU Acute Coronary Syndrome with ST Segment Elevation
3.6. Profilaxis
3.7. Re-Challenge with 5-FU
4. Take Home Message
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| Ach | Acethilcoline |
| ACS | Acute coronary syndrome |
| Cath lab | Catheterization laboratory |
| CCBs | Calcium channel blockers |
| DPYD | Dihydropyrimidine dehydrogenase |
| ED | Emergency Department |
| EF | Ejection fraction |
| EKG | Electrocardiogram |
| ER | Ergonovine |
| FDA | Food and Drug Administration |
| ICCU | Intensive Cardiac Care Unit |
| LBBB | Left bundle branch block |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| ROS | Reactive oxygen species |
| STEMI: | ST-segment elevation myocardial infarction |
References
- Di Paolo, A.; Danesi, R.; Falcone, A.; Cionini, L.; Vannozzi, F.; Masi, G.; Allegrini, G.; Mini, E.; Bocci, G.; Conte, P.F.; et al. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann. Oncol. 2001, 12, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef] [Green Version]
- Casale, J.; Crane, J.S. Fluorouracil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Jensen, S.A.; Sorensen, J.B. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother. Pharmacol. 2006, 58, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Etienne, M.C.; Pierrefite, V.; Barberi-Heyob, M.; Deporte-Fety, R.; Renee, N. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br. J. Cancer 1999, 79, 627–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iachetta, F.; Bonelli, C.; Romagnani, A.; Zamponi, R.; Tofani, L.; Farnetti, E.; Nicoli, D.; Damato, A.; Banzi, M.; Casali, B.; et al. The clinical relevance of multiple DPYD polymorphisms on patients candidate for fluoropyrimidine based-chemotherapy. An Italian case-control study. Br. J. Cancer 2019, 120, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.M.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Deenen, M.J.; Meulendijks, D.; Cats, A.; Sechterberger, M.K.; Severens, J.L.; Boot, H.; Smits, P.H.; Rosing, H.; Mandigers, C.M.; Soesan, M.; et al. Upfront Genotyping of DPYD*2A to Individualize Fluoropyrimidine Therapy: A Safety and Cost Analysis. J. Clin. Oncol. 2016, 34, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kuilenburg, A.B.; Muller, E.W.; Haasjes, J.; Meinsma, R.; Zoetekouw, L.; Waterham, H.R.; Baas, F.; Richel, D.J.; van Gennip, A.H. Lethal outcome of a patient with a complete dihydropyrimidine dehydrogenase (DPD) deficiency after administration of 5-fluorouracil: Frequency of the common IVS14+1G>A mutation causing DPD deficiency. Clin. Cancer Res. 2001, 7, 1149–1153. [Google Scholar]
- Ray, J.C.; Cho, P.; Dragon, M.; Graham, C.G. A Case of 5-Fluorouracil-Induced Cardiac Arrest. J. Emerg. Med. 2016, 50, e1–e6. [Google Scholar] [CrossRef]
- Peng, J.; Dong, C.; Wang, C.; Li, W.; Yu, H.; Zhang, M.; Zhao, Q.; Zhu, B.; Zhang, J.; Li, W.; et al. Cardiotoxicity of 5-fluorouracil and capecitabine in Chinese patients: A prospective study. Cancer Commun. 2018, 38, 22. [Google Scholar] [CrossRef] [Green Version]
- Tsavaris, N.; Kosmas, C.; Vadiaka, M.; Efremidis, M.; Zinelis, A.; Beldecos, D.; Sakelariou, D.; Koufos, C.; Stamatelos, G. Cardiotoxicity following different doses and schedules of 5-fluorouracil administration for malignancy—A survey of 427 patients. Med. Sci. Monit. 2002, 8, PI51–PI57. [Google Scholar]
- Labianca, R.; Beretta, G.; Clerici, M.; Fraschini, P.; Luporini, G. Cardiac toxicity of 5-fluorouracil: A study on 1083 patients. Tumori 1982, 68, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, J.J.; Simkens, L.H.; Mol, L.; Kok, W.E.; Koopman, M.; Punt, C.J. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: A retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur. J. Cancer 2017, 76, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pottage, A.; Holt, S.; Ludgate, S.; Langlands, A.O. Fluorouracil cardiotoxicity. Br. Med. J. 1978, 1, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyss, A.; Milano, G.; Schneider, M.; Demard, F. Circulating drug levels in patients presenting cardiotoxicity to 5-FU. Eur. J. Cancer Clin. Oncol. 1988, 24, 1675–1676. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Xiang, D.; Yang, J.; Liu, D.; Ren, X.; Zhang, C. Assessment of dose-response relationship of 5-fluorouracil to murine intestinal injury. Biomed. Pharm. 2018, 106, 910–916. [Google Scholar] [CrossRef]
- Dechant, C.; Baur, M.; Bock, R.; Czejka, M.; Podczeck-Schweighofer, A.; Dittrich, C.; Christ, G. Acute Reversible Heart Failure Caused by Coronary Vasoconstriction due to Continuous 5-Fluorouracil Combination Chemotherapy. Case Rep. Oncol. 2012, 5, 296–301. [Google Scholar] [CrossRef]
- Yeddi, A.; Adam, O.; Khalid, M.; Farah, Y.; Yeddi, O.; Shereef, H.; Yassin, A.; Yeddi, M.; Ghandour, M.; Omer, M.; et al. 5-Fluorouracil-Associated Cardiogenic Shock. Am. J. Ther. 2019. [Google Scholar] [CrossRef]
- Basselin, C.; Fontanges, T.; Descotes, J.; Chevalier, P.; Bui-Xuan, B.; Feinard, G.; Timour, Q. 5-Fluorouracil-induced Tako-Tsubo-like syndrome. Pharmacotherapy 2011, 31, 226. [Google Scholar] [CrossRef]
- Iskandar, M.Z.; Quasem, W.; El-Omar, M. 5-Fluorouracil cardiotoxicity: Reversible left ventricular systolic dysfunction with early detection. BMJ Case Rep. 2015. [Google Scholar] [CrossRef] [Green Version]
- Mishra, T.; Shokr, M.; Ahmed, A.; Afonso, L. Acute reversible left ventricular systolic dysfunction associated with 5-fluorouracil therapy: A rare and increasingly recognised cardiotoxicity of a commonly used drug. BMJ Case Rep. 2019, 12, e230499. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.A.; Hasbak, P.; Mortensen, J.; Sorensen, J.B. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J. Clin. Oncol. 2010, 28, 5280–5286. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Shah, M.M.; Shah, A.R. Fluoropyrimidine-associated cardiotoxicity: Revisited. Expert Opin. Drug Saf. 2009, 8, 191–202. [Google Scholar] [CrossRef]
- Hrovatin, E.; Viel, E.; Lestuzzi, C.; Tartuferi, L.; Zardo, F.; Brieda, M.; Dametto, E.; Piazza, R.; Antonini-Canterin, F.; Vaccher, E.; et al. Severe ventricular dysrhythmias and silent ischemia during infusion of the antimetabolite 5-fluorouracil and cis-platin. J. Cardiovasc. Med. (Hagerstown) 2006, 7, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.; Kloner, R.A.; Ensley, J.; al-Sarraf, M.; Revels, S.; Olivenstein, A.; Bhasin, S.; Kerpel-Fronious, S.; Turi, Z.G. Continuous ambulatory ECG monitoring during fluorouracil therapy: A prospective study. J. Clin. Oncol. 1989, 7, 509–514. [Google Scholar] [CrossRef]
- Depetris, I.; Marino, D.; Bonzano, A.; Cagnazzo, C.; Filippi, R.; Aglietta, M.; Leone, F. Fluoropyrimidine-induced cardiotoxicity. Crit. Rev. Oncol. Hematol. 2018, 124, 1–10. [Google Scholar] [CrossRef]
- Freeman, N.J.; Costanza, M.E. 5-Fluorouracil-associated cardiotoxicity. Cancer 1988, 61, 36–45. [Google Scholar] [CrossRef]
- Layoun, M.E.; Wickramasinghe, C.D.; Peralta, M.V.; Yang, E.H. Fluoropyrimidine-Induced Cardiotoxicity: Manifestations, Mechanisms, and Management. Curr. Oncol. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, L.; Barry, S.; Debourdeau, P.; Cohen, A.; Tournigand, C. [Cardiotoxicity of 5-fluorouracil]. Bull. Cancer 2004, 91 (Suppl. 3), 154–158. [Google Scholar]
- Alter, P.; Herzum, M.; Soufi, M.; Schaefer, J.R.; Maisch, B. Cardiotoxicity of 5-fluorouracil. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 1–5. [Google Scholar] [CrossRef]
- Atar, A.; Korkmaz, M.E.; Ozin, B. Two cases of coronary vasospasm induced by 5-fluorouracil. Anadolu Kardiyol. Derg. 2010, 10, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Camaro, C.; Danse, P.W.; Bosker, H.A. Acute chest pain in a patient treated with capecitabine. Neth. Heart J. 2009, 17, 288–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, R.; Saadat, H.; Taherkhani, M.; Movahed, M.R. Angina induced by 5-fluorouracil infusion in a patient with normal coronaries. Am. Heart Hosp. J. 2010, 8, E111–E112. [Google Scholar] [CrossRef] [PubMed]
- Luwaert, R.J.; Descamps, O.; Majois, F.; Chaudron, J.M.; Beauduin, M. Coronary artery spasm induced by 5-fluorouracil. Eur. Heart J. 1991, 12, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.R.; Shah, A.; Rather, A. Ventricular fibrillation as a likely consequence of capecitabine-induced coronary vasospasm. J. Oncol. Pharm. Pract. 2012, 18, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Vaccher, E.; Talamini, R.; Lleshi, A.; Meneguzzo, N.; Viel, E.; Scalone, S.; Tartuferi, L.; Buonadonna, A.; Ejiofor, L.; et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: An underestimated risk. Ann. Oncol. 2014, 25, 1059–1064. [Google Scholar] [CrossRef]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lestuzzi, C.; Tartuferi, L.; Corona, G. Capecitabine (and 5 fluorouracil) cardiotoxicity. Metabolic considerations. Breast J. 2011, 17, 564–565. [Google Scholar] [CrossRef]
- Kleiman, N.S.; Lehane, D.E.; Geyer, C.E., Jr.; Pratt, C.M.; Young, J.B. Prinzmetal’s angina during 5-fluorouracil chemotherapy. Am. J. Med. 1987, 82, 566–568. [Google Scholar] [CrossRef]
- Mosseri, M.; Fingert, H.J.; Varticovski, L.; Chokshi, S.; Isner, J.M. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993, 53, 3028–3033. [Google Scholar] [PubMed]
- Salepci, T.; Seker, M.; Uyarel, H.; Gumus, M.; Bilici, A.; Ustaalioglu, B.B.; Ozturk, A.; Sonmez, B.; Orcun, A.; Ozates, M.; et al. 5-Fluorouracil induces arterial vasoconstrictions but does not increase angiotensin II levels. Med. Oncol. 2010, 27, 416–420. [Google Scholar] [CrossRef]
- Thyss, A.; Gaspard, M.H.; Marsault, R.; Milano, G.; Frelin, C.; Schneider, M. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann. Oncol. 1992, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Hillis, L.D. Prinzmetal’s variant angina. Clin. Cardiol. 1998, 21, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Sasson, Z.; Morgan, C.D.; Wang, B.; Thomas, G.; MacKenzie, B.; Platts, M.E. 5-Fluorouracil related toxic myocarditis: Case reports and pathological confirmation. Can. J. Cardiol. 1994, 10, 861–864. [Google Scholar]
- Calik, A.N.; Celiker, E.; Velibey, Y.; Cagdas, M.; Guzelburc, O. Initial dose effect of 5-fluorouracil: Rapidly improving severe, acute toxic myopericarditis. Am. J. Emerg. Med. 2012, 30, 257.E1–257.E3. [Google Scholar] [CrossRef]
- Dalzell, J.R.; Samuel, L.M. The spectrum of 5-fluorouracil cardiotoxicity. Anticancer Drugs 2009, 20, 79–80. [Google Scholar] [CrossRef]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sessa, F.; Noonan, D.M.; et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Sueda, S.; Kohno, H.; Ochi, T.; Uraoka, T.; Tsunemitsu, K. Overview of the pharmacological spasm provocation test: Comparisons between acetylcholine and ergonovine. J. Cardiol. 2017, 69, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Eskilsson, J.; Albertsson, M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncol. 1990, 29, 1001–1003. [Google Scholar] [CrossRef]
- Patel, B.; Kloner, R.A.; Ensley, J.; Al-Sarraf, M.; Kish, J.; Wynne, J. 5-Fluorouracil cardiotoxicity: Left ventricular dysfunction and effect of coronary vasodilators. Am. J. Med. Sci. 1987, 294, 238–243. [Google Scholar] [CrossRef]
- Oleksowicz, L.; Bruckner, H.W. Prophylaxis of 5-fluorouracil-induced coronary vasospasm with calcium channel blockers. Am. J. Med. 1988, 85, 750–751. [Google Scholar] [CrossRef]
- Clasen, S.C.; Ky, B.; O’Quinn, R.; Giantonio, B.; Teitelbaum, U.; Carver, J.R. Fluoropyrimidine-induced cardiac toxicity: Challenging the current paradigm. J. Gastrointest Oncol. 2017, 8, 970–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosy, A.P.; Kunz, P.L.; Fisher, G.A.; Witteles, R.M. Capecitabine-induced chest pain relieved by diltiazem. Am. J. Cardiol. 2012, 110, 1623–1626. [Google Scholar] [CrossRef]
- Redman, J.M.; Rhea, L.P.; Brofferio, A.; Whelpley, M.; Gulley, J.L.; Gatti-Mays, M.E.; McMahon, S.; Cordes, L.M.; Strauss, J. Successful 5-fluorouracil (5-FU) infusion re-challenge in a metastatic colorectal cancer patient with coronary artery disease who experienced symptoms consistent with coronary vasospasm during first 5-FU infusion. J. Gastrointest. Oncol. 2019, 10, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Vargo, C.A.; Blazer, M.; Reardon, J.; Gulati, M.; Bekaii-Saab, T. Successful Completion of Adjuvant Chemotherapy in a Patient With Colon Cancer Experiencing 5-Fluorouracil-Induced Cardiac Vasospasm. Clin. Colorectal Cancer 2016, 15, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Deboever, G.; Hiltrop, N.; Cool, M.; Lambrecht, G. Alternative treatment options in colorectal cancer patients with 5-fluorouracil- or capecitabine-induced cardiotoxicity. Clin. Colorectal Cancer 2013, 12, 8–14. [Google Scholar] [CrossRef] [Green Version]



| Luwaert et al. [36] | Camaro et al. [34] | Dalzell and Samuel [48] | Atar et al. Patient 1 [33] | Atar et al. Patient 2 [33] | Shah et al. [37] | Dechant et al. [18] | |
|---|---|---|---|---|---|---|---|
| AGE/GENDER | 70/M | 61/M | 54/M | 40/F | 63/M | 28/M | 51/M |
| PRIOR CARDIAC HYSTORY | None | Mild coronary artery disease | None | None | Unspecified coronary artery disease | None | None |
| CARDIAC RISK FACTORS | Smoke, arterial hypertension | Not reported | Not reported | Not reported | Not reported | None | Smoke, arterial hypertension, PAD, hyperlipidemia |
| CANCER | Squamous carcinoma of the palate | Metastatic colorectal cancer | Colon adenocarcinoma | Adenocarcinoma of the cecum | Adenocarcinoma of the duodenum | Metastatic colorectal cancer | Rectal cancer |
| ADDITIONAL CHEMOTHERAPY | Carboplatin | None | Oxaliplatin, leucovorin | Folinic acid | Folinic acid | None | Non reported |
| MODE OF ADIMINISTRATION | 5-FU infusion | Oral capecitabine | 5-FU infusion | 5-FU infusion | 5-FU infusion | Oral capecitabine | 5-FU bolus + infusion |
| DOSE | 1000 mg/m2/day | 1500 mg/m2 twice daily | NR | 425 mg/m2/day | 425 mg/m2/day | 1250 mg/m2 twice daily | 400 mg/m2 iv bolus followed by 2400 mg/m2 iv infusion. |
| SYMPTOMS | Angina pectoris | Retrosternal chest pain | Typical chest pain | Chest pain | Chest pain | Cardiac arrest (ventricular fibrillation) | Typical chest pain |
| TIMING OF ONSET SYMPTOMS | Day 3 | Day 1 | 20 h into the infusion | Day 3 | Day 3 | 5 of a total of 6 cycles of chemotherapy | Day 2 |
| EKG | ST-segment elevation in inferolateral leads | ST-segment elevation in inferolateral leads and peaked T-waves | Lateral ST elevation with reciprocal change alternating with intermittent left bundle branch block | ST-segment elevation in leads II, III, aVF, V5 and V6 | ST-segment elevation in leads II, III, aVF, V5 and V6 | Post-ROSC:ST segment elevation in the inferolateral leads | Significant ST elevations and prominent T waves in almost all leads (I–III, aVF and V2–V6) |
| TROPONIN | Not reported | Normal | Elevated | Normal | Normal | Elevated | Not reported |
| ECHO | Normal | Normal | EF 30%, global hypokinesis | Normal | Normal | Normal | EF 24%, global hypokinesis |
| CATH | Normal coronaries, ergonovine test + for spasm | No changes in comparison with the previous angiography | Normal coronaries | Normal coronaries, cold pressor test + for spasm | Severe multivessel coronary artery disease, cold pressor test | Normal coronaries | Generally reduced coronary flow. after 2 days normal coronaries. |
| INTERVENTION | Diltiazem and nitrate | Nifedipine | Ramipril, Metoprolol | Diltiazem | Diltiazem and Nitrate | Verapamil, defibrillator | ACE inhibitor, Verapamil, diuretics |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksova, A.; Gagno, G.; Pierri, A.; Todaro, C.; Fluca, A.L.; Orlando, V.; Guglielmi, A.; Beltrami, A.P.; Sinagra, G. What the Cardiologist Needs to Consider in the Management of Oncologic Patients with STEMI-Like Syndrome: A Case Report and Literature Review. Pharmaceuticals 2021, 14, 563. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060563
Aleksova A, Gagno G, Pierri A, Todaro C, Fluca AL, Orlando V, Guglielmi A, Beltrami AP, Sinagra G. What the Cardiologist Needs to Consider in the Management of Oncologic Patients with STEMI-Like Syndrome: A Case Report and Literature Review. Pharmaceuticals. 2021; 14(6):563. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060563
Chicago/Turabian StyleAleksova, Aneta, Giulia Gagno, Alessandro Pierri, Carla Todaro, Alessandra Lucia Fluca, Valentina Orlando, Alessandra Guglielmi, Antonio Paolo Beltrami, and Gianfranco Sinagra. 2021. "What the Cardiologist Needs to Consider in the Management of Oncologic Patients with STEMI-Like Syndrome: A Case Report and Literature Review" Pharmaceuticals 14, no. 6: 563. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060563






