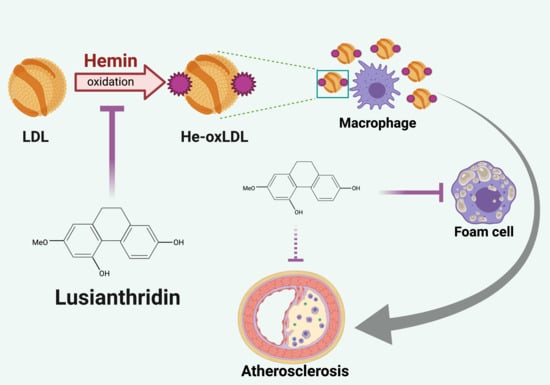
Protective Effect of Lusianthridin on Hemin-Induced Low-Density Lipoprotein Oxidation
Abstract
:1. Introduction
2. Results
2.1. Effect of Lusianthridin on TBARs Formation
2.2. Effect of Lusianthridin on Relative Electrophoretic Mobility
2.3. Effect of Lusianthridin on Lipid Level
2.4. Effect of Lusianthridin on RAW 264.7 Macrophage Cell Viability
2.5. Effect of Lusianthridin on Foam Cell Formation in RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of LDL
4.3. Hemin-Induced LDL Oxidation
4.4. Formation of Thiobarbituric Reactive Substance (TBARs)
4.5. Relative Electrophoretic Mobility (REM) of LDL
4.6. Determination of the Level of Lipid
4.7. Cell Culture
4.8. Cell Viability Assay
4.9. Foam Cell Formation Detection
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Nakano, E.; Williamson, M.P.; Williams, N.H.; Powers, H.J. Copper-mediated LDL oxidation by homocysteine and related compounds depends largely on copper ligation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2004, 1688, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, Y.I.; Shaklai, N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1999, 1454, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Phumala, N.; Porasuphatana, S.; Unchern, S.; Pootrakul, P.; Fucharoen, S.; Chantharaksri, U. Hemin: A possible cause of oxidative stress in blood circulation of β-thalassemia/hemoglobin E disease. Free Radic. Res. 2003, 37, 129–135. [Google Scholar] [CrossRef]
- Merchant, R.H.; Someshwar Chate, J.A.; Ahmad, N.; Karnik, A.; Jankaria, B. Evaluation of carotid artery dynamics & correlation with cardiac & hepatic iron in β-thalassaemia patients. Indian J. Med. Res. 2016, 143, 443. [Google Scholar]
- Camejo, G.; Halberg, C.; Manschik-Lundin, A.; Hurt-Camejo, E.; Rosengren, B.; Olsson, H.; Hansson, G.I.; Forsberg, G.-B.; Ylhen, B. Hemin binding and oxidation of lipoproteins in serum: Mechanisms and effect on the interaction of LDL with human macrophages. J. Lipid Res. 1998, 39, 755–766. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low-density lipoprotein. In Free Radicals and Antioxidant Protocols; Springer: Berlin/Heidelberg, Germany, 2010; pp. 403–417. [Google Scholar]
- Luechapudiporn, R.; Morales, N.P.; Fucharoen, S.; Chantharaksri, U. The reduction of cholesteryl linoleate in lipoproteins: An index of clinical severity in β-thalassemia/Hb E. Clin. Chem. Lab. Med. (CCLM) 2006, 44, 574–581. [Google Scholar] [CrossRef]
- Morales, N.P.; Chunephisal, P.; Janprasit, J.; Ishida, Y.; Luechapudiporn, R.; Yamada, K.I. Kinetics and localisation of haemin-induced lipoprotein oxidation. Free Radic. Res. 2019, 53, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010, 4, 592–638. [Google Scholar]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat. Prod. Commun. 2014, 9, 1934578X1400900625. [Google Scholar] [CrossRef] [Green Version]
- Choonong, R.; Sermpradit, W.; Kitisripanya, T.; Sritularak, B.; Putalun, W. The contents of bibenzyl derivatives, flavonoids and a phenanthrene in selected Dendrobium spp. and the correlation with their antioxidant activity. ScienceAsia 2019, 45, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.-Y.; Wang, J.; Wang, N.-L.; Kitanaka, S.; Yao, X.-S. 9, 10-Dihydrophenanthrene derivatives from Pholidota yunnanensis and scavenging activity on DPPH free radical. J. Asian Nat. Prod. Res. 2007, 9, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chaniad, P.; Morales, N.P.; Rojsitthisak, P.; Luechapudiporn, R. Effects of turmeric extract on hemin-induced low-density lipoprotein oxidation. J. Food Biochem. 2018, 42, e12507. [Google Scholar] [CrossRef]
- Kamido, H.; Kuksis, A.; Marai, L.; Myher, J. Identification of core aldehydes amongin vitro peroxidation products of cholesteryl esters. Lipids 1993, 28, 331–336. [Google Scholar] [CrossRef]
- Steinberg, D. Oxidative modification of LDL and atherogenesis. In Multiple Risk Factors in Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 1998; pp. 141–147. [Google Scholar]
- Yamauchi, R.; Watanabe, S.; Martín, A.S.; Iwamoto, S. Effect of α-tocopherol on the hemin-catalyzed decomposition of 1-palmitoyl-2-linoleoyl-3-sn-phosphatidylcholine 13-hydroperoxide in micelles and liposomes. Chem. Phys. Lipids 2014, 184, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xie, S.; Hong, D.; Ding, Y. An in vitro model of foam cell formation induced by a stretchable microfluidic device. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kisugi, R. Mechanisms of LDL oxidation. Clin. Chim. Acta 2010, 411, 1875–1882. [Google Scholar] [CrossRef]
- Chen, H.Y.; Shiao, M.S.; Huang, Y.L.; Shen, C.C.; Lin, Y.L.; Kuo, Y.H.; Chen, C.C. Antioxidant principles from ephemerantha l onchophylla. J. Nat. Prod. 1999, 62, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Hohmann, J.; Vasas, A. Phenanthrenes: A promising group of plant secondary metabolites. J. Nat. Prod. 2017, 81, 661–678. [Google Scholar] [CrossRef]
- Boudjada, A.; Touil, A.; Bensouici, C.; Bendif, H.; Rhouati, S. Phenanthrene and dihydrophenanthrene derivatives from Dioscorea communis with anticholinesterase, and antioxidant activities. Nat. Prod. Res. 2019, 33, 3278–3282. [Google Scholar] [CrossRef]
- Clifton, P.M.; Nestel, P.J. Influence of gender, body mass index, and age on response of plasma lipids to dietary fat plus cholesterol. Arterioscler. Thromb. 1992, 12, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, T.; Matsushita, S. Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 1980, 15, 137–140. [Google Scholar] [CrossRef]
- Suwannasual, U.; Pengsuparp, T.; Luechapudiporn, R. Protective effect of deferiprone on 7-ketocholesterol formation in hemin-induced LDL oxidation. Thai J. Pharm. Sci. 2014, 38, 14–20. [Google Scholar]
- Kritharides, L.; Jessup, W.; Gifford, J.; Dean, R. A method for defining the stages of low-density lipoprotein oxidation by the separation of cholesterol and cholesteryl ester-oxidation products using HPLC. Anal. Biochem. 1993, 213, 79–89. [Google Scholar] [CrossRef]
- Winikoff, S.E.; Zeh, H.J.; DeMarco, R.; Lotze, M.T. Cytolytic assays. In Measuring Immunity: Basic Science and Clinical Practice; Elsevier Academic Press: Cambridge, MA, USA, 2011; p. 343. [Google Scholar]
- Xu, S.; Huang, Y.; Xie, Y.; Lan, T.; Le, K.; Chen, J.; Chen, S.; Gao, S.; Xu, X.; Shen, X.; et al. Evaluation of foam cell formation in cultured macrophages: An improved method with Oil Red O staining and DiI-oxLDL uptake. Cytotechnology 2010, 62, 473–481. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thant, S.W.; Morales, N.P.; Buranasudja, V.; Sritularak, B.; Luechapudiporn, R. Protective Effect of Lusianthridin on Hemin-Induced Low-Density Lipoprotein Oxidation. Pharmaceuticals 2021, 14, 567. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060567
Thant SW, Morales NP, Buranasudja V, Sritularak B, Luechapudiporn R. Protective Effect of Lusianthridin on Hemin-Induced Low-Density Lipoprotein Oxidation. Pharmaceuticals. 2021; 14(6):567. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060567
Chicago/Turabian StyleThant, Su Wutyi, Noppawan Phumala Morales, Visarut Buranasudja, Boonchoo Sritularak, and Rataya Luechapudiporn. 2021. "Protective Effect of Lusianthridin on Hemin-Induced Low-Density Lipoprotein Oxidation" Pharmaceuticals 14, no. 6: 567. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060567