Chronic Red Bull Consumption during Adolescence: Effect on Mesocortical and Mesolimbic Dopamine Transmission and Cardiovascular System in Adult Rats
Abstract
:1. Introduction
2. Results
2.1. Consummatory and Metabolic Data
2.1.1. Fluid Intake in Control and Treated Rats during the Dark and the Light Period of the R Week and the 4 Weeks of Treatment
2.1.2. Amount of Food Eaten by Control and RB-Treated Rats during the R Week and the 4 Weeks of Treatment
2.1.3. Weight of Control and RB-Treated Rats during the R Week and the 4 Weeks of Treatment
2.1.4. Levels of Fasting Blood Sugar in Control and RB-Treated Rats during the R Week and the 4 Weeks of Treatment
2.2. Energy Intake by RB-Treated and Control Rats during the 4 Weeks of Treatment
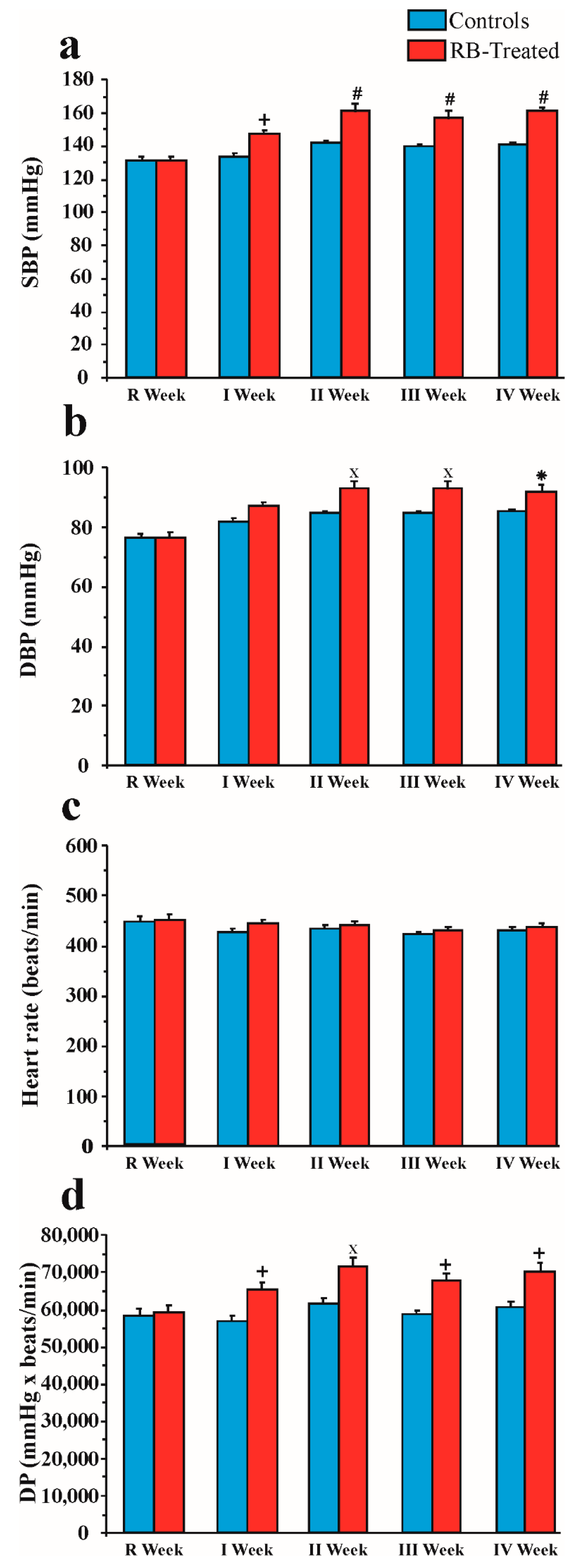
2.3. Effect of Chronic Red Bull Consumption on Blood Pressure and Heart Rate
2.3.1. SBP
2.3.2. DBP
2.3.3. Heart Rate
2.3.4. DP
2.4. Microdialysis and Taste Reactivity Experiments
2.4.1. NAc Shell and Core DA Responsiveness and Taste Reactions after Repeated Intraoral RB Administration in Treated and Control Animals
2.4.2. Effect of Repeated Intraoral RB Infusion on DA Transmission in the Medial PFCX and on Taste Reactions
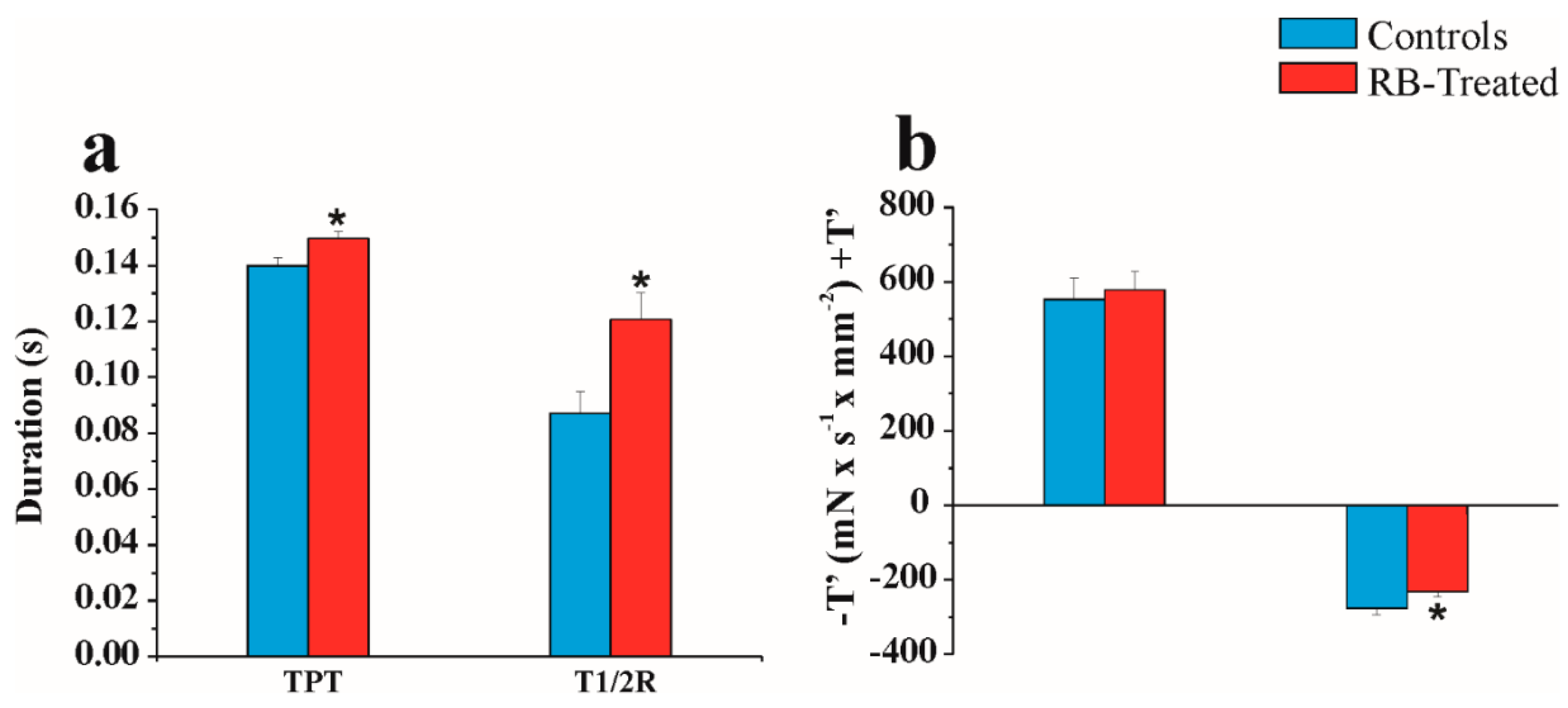
2.5. Physiological “In Vitro” Study
3. Discussion
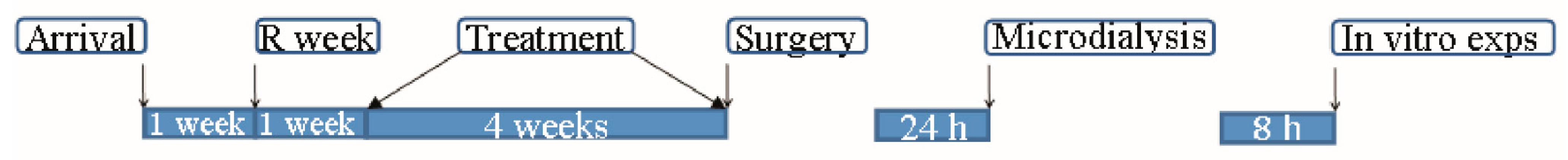
4. Materials and Methods
4.1. Animals
4.2. Red Bull
Red Bull Administration
4.3. Hemodynamic Measurements
- (1)
- Habituation to the restraint procedure.
- (1a)
- Habituation period outside the heated chamber: the rats were immobilized in a plastic holder (6 × 20 × 6 (h) cm) and kept at room temperature for 10 min.
- (1b)
- Habituation period inside the heated chamber: the rats, immobilized in a plastic holder, were moved to the heated chamber (with the temperature kept at 38 °C); a period of acclimatization to the heated chamber of approximately 10 min was observed before the blood pressure recording was started.
- (2)
- Habituation to the pneumatic pulse sensor chamber: a cuff with a pneumatic pulse sensor was attached to the tail of each rat at the beginning of the 10 min habituation period inside the heated chamber.
4.4. Surgery
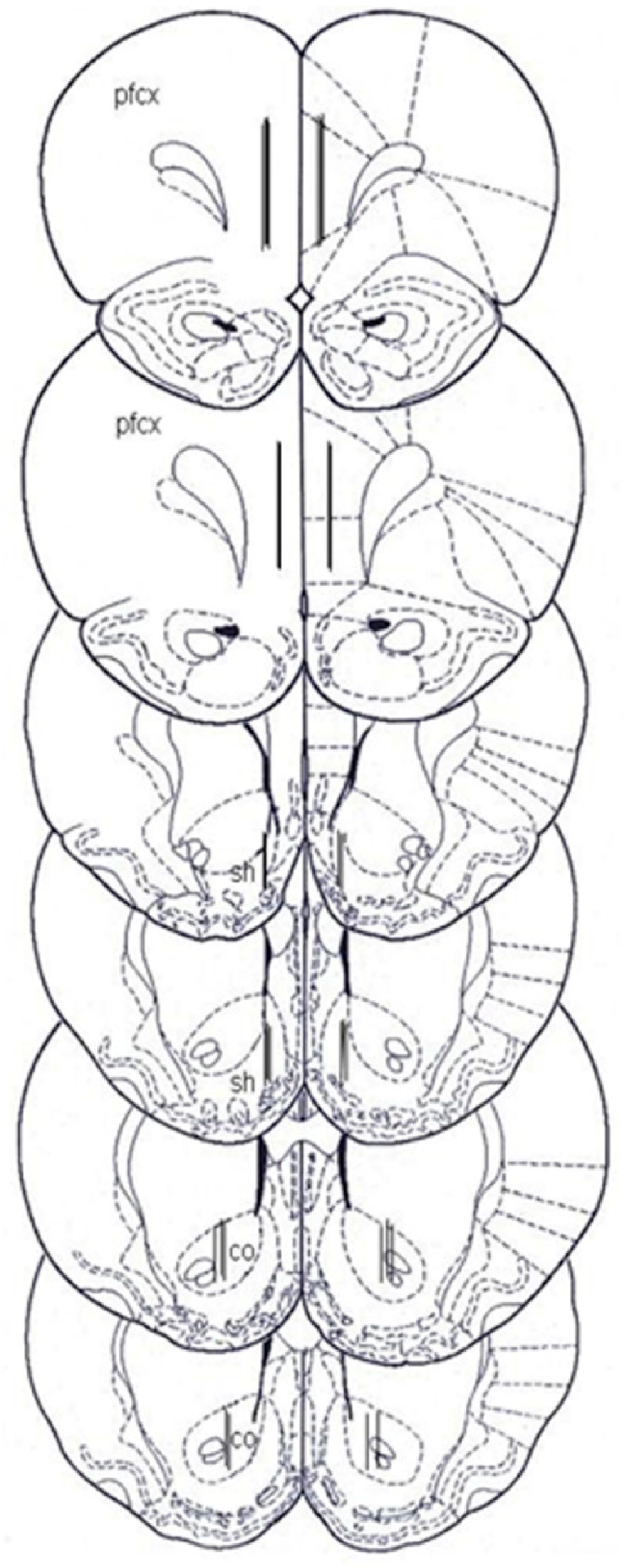
4.5. Microdialysis
4.5.1. Probe and Oral Catheter Preparation
4.5.2. Microdialysis Experiments
4.6. Histology
4.7. Physiology General Procedures
4.7.1. Papillary Muscle Preparation
4.7.2. Papillary Muscle Mechanical and Energetic Parameters
4.7.3. Crossbridge Characteristics
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated energy drinks a growing problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degirmenci, N.; Fossum, I.N.; Strand, T.A.; Vaktskjold, A.; Holten-Andersen, M.N. Consumption of energy drinks among adolescents in Norway: A cross-sectional study. BMC Public Health 2018, 18, 1391. [Google Scholar] [CrossRef] [PubMed]
- Mansour, B.; Amarah, W.; Nasralla, E.; Nael, E. Energy drinks in children and adolescents: Demographic data and immediate effects. Eur. J. Pediatr. 2019, 178, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.; Morlock, G. Simultaneous determination of riboflavin, pyridoxine, nicotinamide, caffeine and taurine in energy drinks by planar chromatography-multiple detection with confirmation by electrospray ionization mass spectrometry. J. Chromatogr. A 2006, 1131, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Iyadurai, S.; Chung, S. New-onset seizures in adults: Possible association with consumption of popular energy drinks. Epilepsy Behav. 2007, 10, 504–508. [Google Scholar] [CrossRef]
- Ruiz, L.D.; Scherr, R.E. Risk of Energy Drink Consumption to Adolescent Health. Am. J. Lifestyle Med. 2018, 13, 22–25. [Google Scholar] [CrossRef]
- Temple, J.L. Review: Trends, Safety, and Recommendations for Caffeine Use in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Worrall, B.B.; Phillips, C.D.; Henderson, K.K. Herbal energy drinks, phenylpropanoid compounds, and cerebral vasculopathy. Neurology 2005, 65, 1137–1138. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Viale, C.I.; Kapczinski, F. Mania associated with an energy drink: The possible role of caffeine, taurine, and inositol. Can. J. Psychiatry 2001, 46, 454–455. [Google Scholar] [CrossRef] [Green Version]
- Quadri, S.; Harding, L.; Lillig, M. An Energy Drink–Induced Manic Episode in an Adolescent. Prim. Care Companion CNS Disord. 2018, 20, 18l02318. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Italiano, D.; Gervasi, G.; Bramanti, P. Single tonic–clonic seizure after energy drink abuse. Epilepsy Behav. 2012, 23, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.E.; Kado, H.S.; Samson, R.; Miller, A.B. A case of caffeine-induced coronary artery vasospasm of a 17-year-old male. Cardiovasc. Toxicol. 2012, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.J.; Alford, K. Cardiac arrest in a young man following excess consumption of caffeinated “energy drinks”. Med. J. Aust. 2009, 190, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Energy drinks and myocardial ischemia: A review of case reports. Cardiovasc. Toxicol. 2016, 16, 207–212. [Google Scholar] [CrossRef]
- Dikici, S.; Saritas, A.; Besir, F.H.; Tasci, A.H.; Kandiset, H. Do energy drinks cause epileptic seizure and ischemic stroke? Am. J. Emerg. Med. 2013, 31, 274.e1–274.e4. [Google Scholar] [CrossRef]
- Grasser, E.K.; Yepuri, G.; Dulloo, A.G.; Montani, J.P. Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults: A randomized cross-over study. Eur. J. Nutr. 2014, 53, 1561–1571. [Google Scholar] [CrossRef] [Green Version]
- Basrai, M.; Schweinlin, A.; Menzel, J.; Mielke, H.; Weikert, C.; Dusemund, B.; Putze, K.; Watzl, B.; Lampen, A.; Bischoff, S.C. Energy Drinks Induce Acute Cardiovascular and Metabolic Changes Pointing to Potential Risks for Young Adults: A Randomized Controlled Trial. J. Nutr. 2019, 149, 441–450. [Google Scholar] [CrossRef]
- Ebuehi, O.A.; Ajayi, O.E.; Onyeulor, A.L.; Awelimobor, D. Effects of oral administration of energy drinks on blood chemistry, tissue histology and brain acetylcholine in rabbits. Niger. Q. J. Hosp. Med. 2011, 21, 29–34. [Google Scholar]
- Munteanu, C.; Rosioru, C.; Tarba, C.; Lang, C. Long-term consumption of energy drinks induces biochemical and ultrastructural alterations in the heart muscle. Anatol. J. Cardiol. 2018, 19, 326–333. [Google Scholar] [CrossRef]
- Diaz, A.; Treviño, S.; Guevara, J.; Muñoz-Arenas, G.; Brambila, E.; Espinosa, B.; Moreno-Rodríguez, A.; Lopez-Lopez, G.; Peña-Rosas, U.; Venegas, B.; et al. Energy Drink Administration in Combination with Alcohol Causes an Inflammatory Response and Oxidative Stress in the Hippocampus and Temporal Cortex of Rats. Oxidative Med. Cell. Longev. 2016, 8725354. [Google Scholar] [CrossRef] [Green Version]
- Di Chiara, G.; Bassareo, V. Reward system and addiction: What dopamine does and doesn’t do. Curr. Opin. Pharmacol. 2007, 7, 69–76, Erratum in Curr. Opin. Pharmacol. 2007, 7, 233. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 1997, 17, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Bassareo, V.; Di Chiara, G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 1999, 89, 637–641. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 1999, 11, 4389–4397. [Google Scholar] [CrossRef]
- Bassareo, V.; De Luca, M.A.; Aresu, M.; Aste, A.; Ariu, T.; Di Chiara, G. Differential adaptive properties of accumbens shell dopamine responses to ethanol as a drug and as a motivational stimulus. Eur. J. Neurosci. 2003, 17, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Gambarana, C.; Masi, F.; Leggio, B.; Grappi, S.; Nanni, G.; Scheggi, S.; De Montis, M.G.; Tagliamonte, A. Acquisition of a palatable-food-sustained appetitive behavior in satiated rats is dependent on the dopaminergic response to this food in limbic areas. Neuroscience 2003, 121, 179–187. [Google Scholar] [CrossRef]
- Rada, P.; Avena, N.M.; Hoebel, B.G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005, 134, 737–744. [Google Scholar] [CrossRef]
- Danielli, B.; Scheggi, S.; Grappi, S.; Marchese, G.; De Montis, M.G.; Tagliamonte, A.; Gambarana, C. Modifications in DARPP-32 phosphorylation pattern after repeated palatable food consumption undergo rapid habituation in the nucleus accumbens shell of non-food-deprived rats. J. Neurochem. 2010, 112, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Bassareo, V.; Cucca, F.; Musio, P.; Lecca, D.; Frau, R.; Di Chiara, G. Nucleus accumbens shell and core dopamine responsiveness to sucrose in rats: Role of response contingency and discriminative/conditioned cues. Eur. J. Neurosci. 2015, 41, 802–809. [Google Scholar] [CrossRef]
- Bassareo, V.; Cucca, F.; Frau, R.; Di Chiara, G. Monitoring dopamine transmission in the rat nucleus accumbens shell and core during acquisition of nose-poking for sucrose. Behav. Brain Res. 2015, 287, 200–206. [Google Scholar] [CrossRef]
- Bassareo, V.; Cucca, F.; Frau, R.; Di Chiara, G. Differential activation of accumbens shell and core dopamine by sucrose reinforcement with nose poking and with lever pressing. Behav. Brain Res. 2015, 294, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Pontieri, F.E.; Tanda, G.; Di Chiara, G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the ‘‘shell’’ as compared with the ‘‘core’’ of the rat nucleus accumbens. Proc. Natl. Acad. Sci. USA 1995, 92, 12304–12308. [Google Scholar] [CrossRef] [Green Version]
- Pontieri, F.E.; Tanda, G.; Orzi, F.; Di Chiara, G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996, 382, 255–257. [Google Scholar] [CrossRef]
- Tanda, G.; Pontieri, F.E.; Di Chiara, G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common m1 opioid receptor mechanism. Science 1997, 276, 2048–2050. [Google Scholar] [CrossRef] [Green Version]
- Lecca, D.; Cacciapaglia, F.; Valentini, V.; Di Chiara, G. Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212–2 self-administration. Psychopharmacology 2006, 188, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Cacciapaglia, F.; Valentini, V.; Gronli, J.; Spiga, S.; Di Chiara, G. Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology 2006, 184, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Cacciapaglia, F.; Valentini, V.; Acquas, E.; Di Chiara, G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology 2007, 191, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Valentini, V.; Cacciapaglia, F.; Acquas, E.; Di Chiara, G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: A repeated sampling microdialysis study. Psychopharmacology 2007, 194, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Aragona, B.J.; Cleaveland, N.A.; Stuber, G.D.; Day, J.J.; Carelli, R.M.; Wightman, R.M. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 2008, 28, 8821–8831. [Google Scholar] [CrossRef] [Green Version]
- Wiss, D.A.; Avena, N.; Rada, P. Sugar. Addiction: From Evolution to Revolution. Front. Psychiatry 2018, 9, 545. [Google Scholar] [CrossRef] [Green Version]
- Bassareo, V.; Cucca, F.; Frau, R.; Di Chiara, G. Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration. Front. Behav. Neurosci. 2017, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Di Chiara, G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J. Psychopharmacol. 1998, 12, 54–67. [Google Scholar] [CrossRef]
- Di Chiara, G. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 1999, 375, 13–30. [Google Scholar] [CrossRef]
- Acquas, E.; Tanda, G.; Di Chiara, G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 2002, 27, 182–193. [Google Scholar] [CrossRef] [Green Version]
- De Luca, M.A.; Bassareo, V.; Bauer, A.; Di Chiara, G. Caffeine and accumbens shell dopamine. J. Neurochem. 2007, 103, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Solinas, M.; Ferre, S.; You, Z.B.; Karcz-Kubicha, M.; Popoli, P.; Goldberg, S.R. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J. Neurosci. 2002, 22, 6321–6324. [Google Scholar] [CrossRef] [PubMed]
- Quarta, D.; Ferre, S.; Solinas, M.; You, Z.B.; Hockemeyer, J.; Popoli, P.; Goldberg, S.R. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J. Neurochem. 2004, 88, 1151–1158. [Google Scholar] [CrossRef]
- Galvalisi, M.; Prieto, J.P.; Martínez, M.; Abin-Carriquiry, J.A.; Scorza, C. Caffeine Induces a Stimulant Effect and Increases Dopamine Release in the Nucleus Accumbens Shell Through the Pulmonary Inhalation Route of Administration in Rats. Neurotox. Res. 2017, 31, 90–98. [Google Scholar] [CrossRef]
- Cacciapaglia, F.; Saddoris, M.P.; Wightman, R.; Carelli, R.M. Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose. Neuropharmacology 2012, 62, 2050–2056. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.; Cucca, F.; Frau, R.; Corrias, F.; Schlich, M.; Caboni, P.; Fadda, A.M.; Bassareo, V. Systemic Administration of Orexin a Loaded Liposomes Potentiates Nucleus Accumbens Shell Dopamine Release by Sucrose Feeding. Front. Psychiatry 2018, 9, 640. [Google Scholar] [CrossRef]
- Hajanal, A.; Norgren, R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport 2002, 13, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Rada, P.; Moise, N.; Hoebel, B.G. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience 2006, 139, 813–820. [Google Scholar] [CrossRef]
- Ericsson, M.; Molander, A.; Stomberg, R.; Söderpalm, B. Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. Eur. J. Neurosci. 2006, 23, 3225–3229. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, S.; Volpato, C.; Adinolfi, F.; Gandini, E.; Gentile, E.; Loi, A.; Fioriti, L. Gathering consumption data on specific consumer groups of energy drinks. EFSA Support. Publ. 2013, 10, 394E. Available online: https://www.efsa.europa.eu/en/supporting/pub/en-394.3sa.europa.eu/en/supporting/pub/en-394.3 (accessed on 6 March 2013). [CrossRef] [Green Version]
- Christensen, L.M.; Iversen, J.D.; Biltoft-Jensen, A.; Petersen, M.A.; Søndergaard, A.B.; Matthiessen, J. Consumption of Energy Drinks among 10–35-yr-old Danes (in Danish with an English Summary); National Food Institute, Technical University of Denmark: Lyngby, Denmark, 2014. [Google Scholar]
- Visram, S.; Cheetham, M.; Riby, D.M.; Crossley, S.J.; Lake, A.A. Consumption of energy drinks by children and young people: A rapid review examining evidence of physical effects and consumer attitudes. BMJ Open 2016, 6, e010380. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC5073652/ (accessed on 10 October 2016). [CrossRef] [Green Version]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef] [Green Version]
- Wolk, B.J.; Ganetsky, M.; Babu, K.M. Toxicity of energy drinks. Curr. Opin. Pediatr. 2012, 24, 243–251. [Google Scholar] [CrossRef]
- Arria, A.M.; Caldeira, K.M.; Kasperski, S.J.; O’Grady, K.E.; Vincent, K.B.; Griffiths, R.R.; Wish, E.D. Increased alcohol consumption, nonmedical prescription drug use, and illicit drug use are associated with energy drink consumption among college students. J. Addict. Med. 2010, 4, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Terry-McElrath, Y.M.; O’Malley, P.M.; Johnston, L.D. Energy drinks, soft drinks, and substance use among United States secondary school students. J. Addict. Med. 2014, 8, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Woolsey, C.L.; Barnes, L.B.; Jacobson, B.H.; Kensinger, W.S.; Barry, A.E.; Beck, N.C.; Resnik, A.G.; Evans, M.W., Jr. Frequency of energy drink use predicts illicit prescription stimulant use. Subst. Abus. 2014, 35, 96–103. [Google Scholar] [CrossRef]
- Arria, A.M.; Caldeira, K.M.; Bugbee, B.A.; Vincent, K.B.; O’Grady, K. Energy drink use trajectories predict substance use outcomes. Drug Alcohol Depend. Coll. Probl. Drug Depend. 2017, 171, e11. [Google Scholar] [CrossRef]
- Jackson, D.B.; Leal, W.E. Energy drink consumption and the perceived risk and disapproval of drugs: Monitoring the Future, 2010–2016. Drug Alcohol Depend. 2018, 188, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.S.; Coleman, C.; Shelton, R.; Heemstra, L.A.; Novak, C.M. Caffeine enhances activity thermogenesis and energy expenditure in rats. Clin. Exp. Pharmacol. Physiol. 2019, 46, 475–482. [Google Scholar] [CrossRef]
- Miles-Chan, J.L.; Charrière, N.; Grasser, E.K.; Montani, J.P.; Dulloo, A.G. The thermic effect of sugar-free Red Bull: Do the non-caffeine bioactive ingredients in energy drinks play a role? Obesity 2015, 3, 16–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acheson, K.J.; Zahorska-Markiewicz, B.; Pittet, P.; Anantharaman, K.; Jéquier, E. Caffeine and coffee: Their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 1980, 33, 989–997. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Seydoux, J.; Girardier, L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: Adenosine antagonism or phosphodiesterase inhibition? Metabolism 1992, 41, 1233–1241. [Google Scholar] [CrossRef]
- Novak, C.M.; Levine, J.A. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J. Neuroendocrinol. 2007, 19, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Westerterp-Plantenga, M.S. Catechin- and caffeine-rich teas for control of body weight in humans. Am. J. Clin. Nutr. 2013, 98 (Suppl. S6), 1682S–1693S. [Google Scholar] [CrossRef] [Green Version]
- Bracale, R.; Petroni, M.L.; Davinelli, S.; Bracale, U.; Scapagnini, G.; Carruba, M.O.; Nisoli, E. Muscle Uncoupling Protein 3 Expression Is Unchanged by Chronic Ephedrine/Caffeine Treatment: Results of a Double Blind, Randomised Clinical Trial in Morbidly Obese Females. PLoS ONE 2014, 9, e98244. [Google Scholar] [CrossRef] [Green Version]
- Miles-Chan, J.L.; Charrière, N.; Grasser, E.K.; Montani, J.P.; Dulloo, A.G. The blood pressure-elevating effect of Red Bull energy drink is mimicked by caffeine but through different hemodynamic pathways. Physiol. Rep. 2015, 3, e12290. [Google Scholar] [CrossRef] [Green Version]
- Flinn, S.; Gregory, J.; McNaughton, L.R.; Tristram, S.; Davies, P. Caffeine ingestion prior to incremental cycling to exhaustion in recreational cyclists. Int. J. Sports Med. 1990, 11, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Nienhueser, J.; Brown, G.A.; Shaw, B.S.; Shaw, I. Effects of Energy Drinks on Metabolism at Rest and During Submaximal Treadmill Exercise in College Age Males. Int. J. Exerc. Sci. 2011, 4, 65–76. [Google Scholar]
- Franks, A.M.; Schmidt, J.M.; McCain, K.R.; Fraer, M. Comparison of the effects of energy drink versus caffeine supplementation on indices of 24-hour ambulatory blood pressure. Ann. Pharmacother. 2012, 46, 192–199. [Google Scholar] [CrossRef]
- Savoca, M.R.; Evans, C.D.; Wilson, M.; Harshfield, G.A.; Ludwig, D.A. The Association of Caffeinated Beverages with Blood Pressure in adolescent. Arch. Pediatr. Adolesc. Med. 2004, 158, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Savoca, M.R.; MacKey, M.L.; Evans, C.D.; Wilson, M.; Ludwig, D.A.; Harshfield, G.A. Association of ambulatory blood pressure and dietary caffeine in adolescents. Am. J. Hypertens. 2005, 18, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Pincomb, G.A.; Lovallo, W.R.; Passey, R.B.; Whitsett, T.L.; Silverstein, S.M.; Wilson, M.F. Effects of caffeine on vascular resistance, cardiac output and myocardial contractility in young men. Am. J. Cardiol. 1985, 56, 119–122. [Google Scholar] [CrossRef]
- Sung, B.H.; Lovallo, W.R.; Pincomb, G.A.; Wilson, M.F. Effects of caffeine on blood pressure response during exercise in normotensive healthy young men. Am. J. Cardiol. 1990, 65, 909–913. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Grasser, E.K.; Miles-Chan, J.L.; Charrière, N.; Loonam, C.R.; Dulloo, A.G.; Montani, J.P. Energy Drinks and Their Impact on the Cardiovascular System: Potential Mechanisms. Adv. Nutr. 2016, 7, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav. 2015, 148, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, T.W.; Griffin, K.A.; Bidani, A.K.; Davisson, R.L.; Hall, J.E. Recommendations for blood pressure measurement in animals. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Virdis, A.; Colucci, R.; Fornai, M.; Blandizzi, C.; Duranti, E.; Pinto, S.; Bernardini, N.; Segnani, C.; Antonioli, L.; Taddei, S.; et al. Cyclooxygenase-2 Inhibition Improves Vascular Endothelial Dysfunction in a Rat Model of Endotoxic Shock: Role of Inducible Nitric-Oxide Synthase and Oxidative Stress. J. Pharmacol. Exp. Ther. 2005, 312, 945–953. [Google Scholar] [CrossRef]
- Mazzone, G.; Morisco, C.; Lembo, V.; D’Argenio, G.; D’Armiento, M.; Rossi, A.; Giudice, C.D.; Trimarco, B.; Caporaso, N.; Morisco, F. Dietary supplementation of vitamin D prevents the development of western diet-induced metabolic, hepatic and cardiovascular abnormalities in rats. United Eur. Gastroenteral J. 2018, 6, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Bassareo, P.P.; Marras, A.R.; Pasqualucci, D.; Mercuro, G. Increased arterial rigidity in children affected by Cushing’s syndrome after successful surgical cure. Cardiol. Young 2010, 20, 610–614. [Google Scholar] [CrossRef]
- Van Vliett, B.N.; Montani, J.P. Baroreflex stabilization of the double product. Am. J. Physiol. 1999, 277, H1679–H1689. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Di Chiara, G.; Tanda, G.; Frau, R.; Carboni, E. On the preferential release of dopamine in the nucleus accumbens by amphetamine: Further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacoly 1993, 112, 98–402. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Bassareo, V.; Di Chiara, G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology 1996, 123, 127–130. [Google Scholar] [CrossRef]
- Grill, H.J.; Norgren, R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978, 143, 263–279. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998, 28, 309–369. [Google Scholar] [CrossRef]
- Eisenberg, E.; Hill, T.L.; Chen, Y.D. Cross-bridge model of muscle contraction: Quantitative analysis. Biophys. J. 1980, 29, 195–225. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, K.A.; Tyska, M.J.; Dupuis, D.E.; Alpert, N.R.; Warshaw, D.M. Kinetics differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J. Physiol. 1999, 519, 669–678. [Google Scholar] [CrossRef]
- Vargiu, R.; Littarru, G.P.; Fraschini, M.; Perinu, A.; Tiano, L.; Capra, A.; Mancinelli, R. Enhancement of shortening velocity, power, and acto-myosin cross bridge (CB) kinetics following long-term treatment with propionyl-L-carnitine, coenzyme Q10 and omega-3 fatty acids in BIO T0-2 cardiomyopathic. Syrian Hamsters papillary muscle. BioFactors 2010, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1938, 126, 136–195. [Google Scholar]
- Coirault, C.; Chemla, D.; Péry-Man, N.; Suard, I.; Lecarpentier, Y. Effects of fatigue on tension-velocity relation of diaphragm. Energetic implication. Am. J. Respir. Crit. Care Med. 1995, 151, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F.; Simmons, R.M. Mechanical properties of the cross-bridges of frog striated muscle. J. Physiol. 1971, 218, 59P–60P. [Google Scholar]
- Blanc, F.X.; Coirault, C.; Salmeros, S.; Chemla, D.; Lecarpentier, Y. Mechanics and crossbridge kinetics of tracheal smooth muscle in two inbred rat strains. Eur. Respir. J 2003, 22, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Housman, J.M.; Williams, R.D. Adolescent Nonmedical Use of Opioids and Alcohol Mixed with Energy Drinks. Am. J. Health Behav. 2018, 42, 65–73. [Google Scholar] [CrossRef]
- Leal, W.E.; Jackson, D.B. The role of energy drink consumption in the intention to initiate marijuana use among adolescents. Addict. Behav. 2019, 93, 240–245. [Google Scholar] [CrossRef]
- Marmorstein, N.R. Investigating associations between caffeinated beverage consumption and later alcohol consumption among early adolescents. Addict. Behav. 2019, 90, 362–368. [Google Scholar] [CrossRef]




| Groups | R Week | I Week | II Week | III Week | IV Week |
|---|---|---|---|---|---|
| Controls | 197.23 ± 7.81 | 231.79 ± 10.14 | 243.90 ± 10.88 | 256.23 ± 12.18 | 274.91 ± 13.17 |
| RB-treated | 205 ± 6.98 | 319.12 ± 14.85 * | 368.60 ± 12.27 * | 395.66 ± 27.71 * | 434.40 ± 31.4 * |
| Ingredients | I Week | II Week | III Week | IV Week |
|---|---|---|---|---|
| Caffeine, mg/pro die | 11.87 ± 0.37 | 14.32 ± 0.43 | 15.24 ± 0.51 | 17.28 ± 0.75 |
| Taurine, mg/pro die | 148.32 ± 4.66 | 179.02 ± 5.44 | 190.50 ± 6.49 | 216.04 ± 9.40 |
| Sugars, mg/prodie | 407.88 ± 12.81 | 492.30 ± 14.96 | 523.88 ± 17.84 | 594.11 ± 25.84 |
| Energy, kcal | 14.54 ± 0.79 | 17.18 ± 0.89 | 16.89 ± 0.79 | 21.75 ± 1.83 |
| Parameters | Controls | RB-Treated |
|---|---|---|
| RT, mN × mm−2 | 9.83 ± 1.01 | 13.85 ± 3.35 |
| P0, mN × mm−2 | 37.73 ± 6.24 | 39.83 ± 5.44 |
| ΔL, L/Lmax | 0.09 ± 0.01 | 0.11 ± 0.01 |
| Vmax, Lmax × s−1 | 0.98 ± 0.08 | 0.97 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargiu, R.; Broccia, F.; Lobina, C.; Lecca, D.; Capra, A.; Bassareo, P.P.; Bassareo, V. Chronic Red Bull Consumption during Adolescence: Effect on Mesocortical and Mesolimbic Dopamine Transmission and Cardiovascular System in Adult Rats. Pharmaceuticals 2021, 14, 609. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070609
Vargiu R, Broccia F, Lobina C, Lecca D, Capra A, Bassareo PP, Bassareo V. Chronic Red Bull Consumption during Adolescence: Effect on Mesocortical and Mesolimbic Dopamine Transmission and Cardiovascular System in Adult Rats. Pharmaceuticals. 2021; 14(7):609. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070609
Chicago/Turabian StyleVargiu, Romina, Francesca Broccia, Carla Lobina, Daniele Lecca, Alessandro Capra, Pier Paolo Bassareo, and Valentina Bassareo. 2021. "Chronic Red Bull Consumption during Adolescence: Effect on Mesocortical and Mesolimbic Dopamine Transmission and Cardiovascular System in Adult Rats" Pharmaceuticals 14, no. 7: 609. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070609






