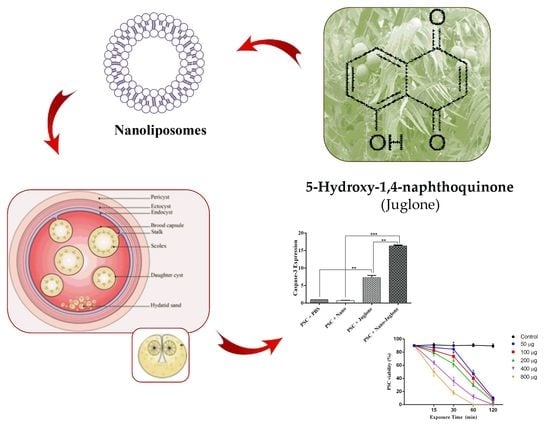
Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces
Abstract
:1. Introduction
2. Results
2.1. Morphology and Zeta Potential Characterization of Liposomal Systems Containing Juglone
2.2. Genotyping of E. granulosus PSCs
2.3. Scolicidal Effects of Juglone and Juglone Nanoliposomes
2.4. Expression of caspase-3 Gene
3. Discussion
4. Materials and Methods
4.1. Preparation of Juglone
4.2. Preparation of Liposomal Systems Containing of Juglone
4.3. Size and Zeta Potential Characterizationof Juglonein Liposomal Systems
4.4. Morphology of Liposomal Systems Containing Juglone
4.5. Collection of E. granulosus PSCs
4.6. Genotyping the PSCs
4.7. Scolicidal Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [Green Version]
- Vuitton, D.A. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: What is the consensus? Expert Rev. Anti-Infect. Ther. 2009, 7, 145–149. [Google Scholar] [CrossRef]
- Rokni, M.B. PP-170 Echinococcosis/hydatidosis in Iran. Int. J. Infect. Dis. 2009, 13, S94–S95. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.; Deplazes, P.; Craig, P.; Gemmell, M.; Gottstein, B.; Heath, D.; Jenkins, D.; Kamiya, M.; Lightowlers, M. Echinococcosis in animals: Clinical aspects, diagnosis, and treatment. In WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; World Organization for Animal Health: Paris, France, 2001; pp. 72–99. [Google Scholar]
- Battelli, G. Evaluation of the economic costs of Echinococcosis. Int. Arch. Hidatid. 1997, 32, 33–37. [Google Scholar]
- da Silva, A.M. Human echinococcosis: A neglected disease. Gastroenterol. Res. Pract. 2010, 35, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Pourseif, M.M.; Yousefpour, M.; Aminianfar, M.; Moghaddam, G.; Nematollahi, A. A multi-method and structure-based in silico vaccine designing against Echinococcus granulosus through investigating enolase protein. BioImpacts 2019, 9, 131–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šarkūnas, M.; Vienažindienė, Ž.; Rojas, C.A.; Radziulis, K.; Deplazes, P. Praziquantel treatment of dogs for four consecutive years decreased the transmission of Echinococcus intermedius G7 to pigs in villages in Lithuania. Food Waterborne Parasitol. 2019, 15, e00043. [Google Scholar] [CrossRef] [PubMed]
- Anvari, D.; Rezaei, F.; Ashouri, A.; Rezaei, S.; Majidiani, H.; Pagheh, A.S.; Shariatzadeh, S.A.; Fotovati, A.; Siyadatpanah, A.; Gholami, S.; et al. Current situation and future prospects of Echinococcus granulosus vaccine candidates: A systematic review. Transbound. Emerg. Dis. 2021, 68, 1080–1096. [Google Scholar] [CrossRef]
- Rouhani, S.; Parvizi, P.; Spotin, A. Using specific synthetic peptide (p176) derived AgB 8/1-kDa accompanied by modified patient’s sera: A novel hypothesis to follow-up of Cystic echinococcosis after surgery. Med. Hypotheses 2013, 81, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Stamatakos, M.; Sargedi, C.; Stefanaki, C.; Safioleas, C.; Matthaiopoulou, I. Anthelminthic treatment: An adjuvant therapeutic strategy against Echinococcus granulosus. Parasitol. Int. 2009, 58, 115–120. [Google Scholar] [CrossRef]
- Adas, G.; Arikan, S.; Kemik, O.; Oner, A.; Sahip, N.; Karatepe, O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study). World J. Gastroenterol. 2009, 15, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Smego, R.A., Jr.; Sebanego, P. Treatment options for hepatic cystic echinococcosis. Int. J. Infect. Dis. 2005, 9, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of Active Antimicrobial Fiber-Based Chitosan Polysaccharide Nanostructures using Electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Kurtyka, R.; Pokora, W.; Tukaj, Z.; Karcz, W. Effects of juglone and lawsone on oxidative stress in maize coleoptile cells treated with IAA. AoB Plants 2016, 8, plw073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blauenburg, B.; Metsä-Ketelä, M.; Klika, K.D. Formation of 5-Hydroxy-3-methoxy-1,4-naphthoquinone and 8-Hydroxy-4-methoxy-1,2-naphthoquinone from Juglone. ISRN Org. Chem. 2012, 2012, 274980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From Oxidative Damage to Cellular and Inter-Cellular Signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef] [Green Version]
- Kot, M.; Karcz, W.; Zaborska, W. 5-Hydroxy-1,4-naphthoquinone (juglone) and 2-hydroxy-1,4-naphthoquinone (lawsone) influence on jack bean urease activity: Elucidation of the difference in inhibition activity. Bioorg. Chem. 2010, 38, 132–137. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Godrati-Azar, Z.; Spotin, A.; Norouzi, R.; Hamishehkar, H.; Nami, S.; Heydarian, P.; Rajabi, S.; Mohammadi, M.; Perez-Cordon, G. Nanostructured lipid carriers of ivermectin as a novel drug delivery system in hydatidosis. Parasites Vectors 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fakhar, M.; Chabra, A.; Rahimi-Esboei, B.; Rezaei, F. In vitro protoscolicidal effects of fungal chitosan isolated from Penicilliumwaksmanii and Penicillium citrinum. J. Parasit. Dis. 2013, 39, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Gholami, S.H.; Rahimi-Esboei, B.; Ebrahimzadeh, M.A.; Pourhajibagher, M. In vitro effect of Sambucus ebulus on scolices of Hydatid cysts. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1760–1765. [Google Scholar] [PubMed]
- Lv, H.; Jiang, Y.; Liao, M.; Sun, H.; Zhang, S.; Peng, X. In vitro and in vivo treatments of Echinococcus granulosus with Huaier aqueous extract and albendazole liposome. Parasitol. Res. 2012, 112, 193–198. [Google Scholar] [CrossRef]
- Kohansal, M.H.; Nourian, A.; Rahimi, M.T.; Daryani, A.; Spotin, A.; Ahmadpour, E. Natural products applied against hydatid cyst protoscolices: A review of past to present. Acta Trop. 2017, 176, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Tappeh, K.H.; Einshaei, A.; Mahmudloo, R.; Mohammadzadeh, H.; Tahermaram, M.; Mousavi, S.J. Effect of Different Concentrations of Hypertonic Saline at Different Times on Protoscoleces of Hydatid Cyst Isolated From Liver and Lung. Turk. J. Parasitol. 2011, 35, 148–150. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, B.; Kong, J.; Cai, H.; Zhao, Y.; Han, X.; Li, F. In vitro protoscolicidal effects of high-intensity focused ultrasound enhanced by a superabsorbent polymer. Parasitol. Res. 2012, 112, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, N.M.; Spotin, A.; Mahami-Oskouei, M.; Ahmadpour, E.; Rostami, A. Recent advances on innate immune pathways related to host–parasite cross-talk in cystic and alveolar echinococcosis. Parasites Vectors 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Moghaddam, S.M.; Picot, S.; Ahmadpour, E. Interactions between hydatid cyst and regulated cell death may provide new therapeutic opportunities. Parasite 2019, 26, 70. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Pan, D.; Bera, A.; Sreevatsava, V.; Bandyopadhyay, S.; Chaudhuri, D.; Kumar, S.; Rana, T.; Das, S.; Suryanaryana, V.; et al. In vitro assessment of praziquantel and a novel nanomaterial against protoscoleces of Echinococcus granulosus. J. Helminthol. 2011, 86, 26–29. [Google Scholar] [CrossRef]
- Hu, H.; Kang, J.; Chen, R.; Mamuti, W.; Wu, G.; Yuan, W. Drug-induced apoptosis of Echinococcus granulosusprotoscoleces. Parasitol. Res. 2011, 109, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.T.; Ahmadpour, E.; Esboei, B.R.; Spotin, A.; Koshki, M.H.K.; Alizadeh, A.; Honary, S.; Barabadi, H.; Mohammadi, M.A. Scolicidal activity of biosynthesized silver nanoparticles against Echinococcus granulosusprotoscolices. Int. J. Surg. 2015, 19, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Akbarzadeh, A.; Spotin, A.; Akbari, N.A.R.; Mahami-Oskouei, M.; Ahmadpour, E. Scolicidal and apoptotic activities of albendazole sulfoxide and albendazole sulfoxide-loaded PLGA-PEG as a novel nanopolymeric particle against Echinococcusgranulosus protoscoleces. Parasitol. Res. 2016, 115, 4595–4603. [Google Scholar] [CrossRef]
- Yetim, I.; Erzurumlu, K.; Hökelek, M.; Baris, S.; Dervisoglu, A.; Polat, C.; Belet, Ü.; Buyukkarabacak, Y.; Güvenli, A. Results of alcohol and albendazole injections in hepatic hydatidosis: Experimental study. J. Gastroenterol. Hepatol. 2005, 20, 1442–1447. [Google Scholar] [CrossRef]
- Ramírez, T.; Benítez-Bribiesca, L.; Ostrosky-Wegman, P.; Herrera, L.A. In Vitro Effects of Albendazole and Its Metabolites on the Cell Proliferation Kinetics and Micronuclei Frequency of Stimulated Human Lymphocytes. Arch. Med. Res. 2001, 32, 119–122. [Google Scholar] [CrossRef]
- Delatour, P.; Parish, R.C.; Gyurik, R.J. Albendazole: A comparison of relay embryotoxicity with embryotoxicity of individual metabolites. Ann. Rech. Veter Ann. Veter Res. 1981, 12, 159–167. [Google Scholar]
- Mohammadi, M.; Pezeshki, A.; Abbasi, M.M.; Ghanbarzadeh, B.; Hamishehkar, H. Vitamin D3-Loaded Nanostructured Lipid Carriers as a Potential Approach for Fortifying Food Beverages; in Vitro and in Vivo Evaluation. Adv. Pharm. Bull. 2017, 7, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghadaszadeh, M.; Khayyati, M.; Spotin, A.; Norouzi, R.; Pagheh, A.S.; Oliveira, S.M.R.; Pereira, M.d.L.; Ahmadpour, E. Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces. Pharmaceuticals 2021, 14, 623. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070623
Moghadaszadeh M, Khayyati M, Spotin A, Norouzi R, Pagheh AS, Oliveira SMR, Pereira MdL, Ahmadpour E. Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces. Pharmaceuticals. 2021; 14(7):623. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070623
Chicago/Turabian StyleMoghadaszadeh, Masoud, Mehdi Khayyati, Adel Spotin, Roghayeh Norouzi, Abdol Sattar Pagheh, Sonia M. R. Oliveira, Maria de Lourdes Pereira, and Ehsan Ahmadpour. 2021. "Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces" Pharmaceuticals 14, no. 7: 623. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070623