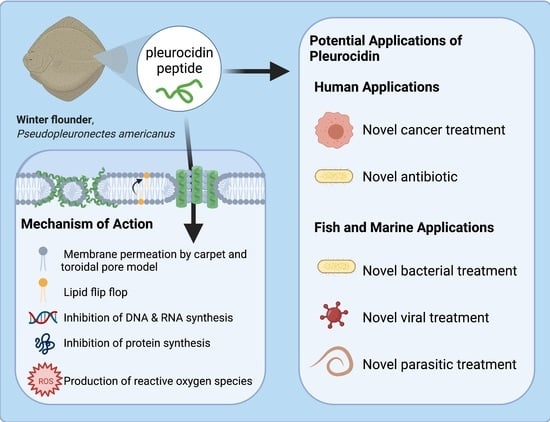
Investigating Potential Applications of the Fish Anti-Microbial Peptide Pleurocidin: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Fish Immunity
3.2. Fish Pathogens
3.3. Pleurocidin
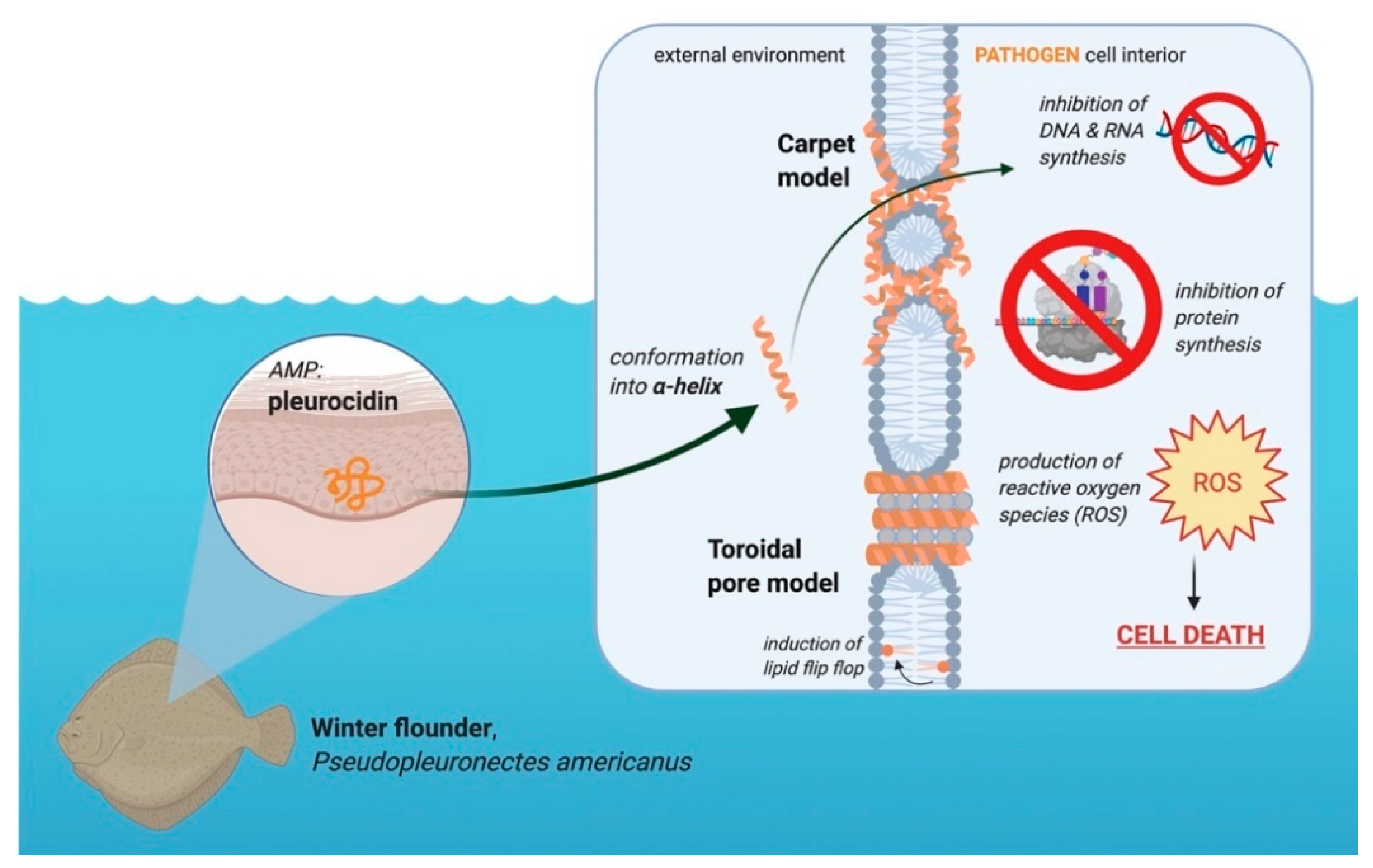
3.4. Pleurocidin’s Mechanism of Action
3.5. Optimization of Pleurocidin
3.6. Potential Clinical Anti-Microbial and Anti-Cancer Applications
3.7. Conservational Applications of Pleurocidin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Chambers, R.; Leggett, W. Size and Age at Metamorphosis in Marine Fishes: An Analysis of Laboratory-Reared Winter Flounder (Pseudopleutonectes americanus) with a Review of Variation in Other Species. Can. J. Fish Aquat. Sci. 2011, 44, 1936–1947. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and Characterization of Pleurocidin, an Antimicrobial Peptide in the Skin Secretions of Winter Flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.J.; Kang, N.H.; Kim, M.K.; Park, J.; Park, E.; Park, G.H.; Kang, T.W.; Na, D.E.; Park, J.B.; Yi, Y.E.; et al. Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus. Microb. Pathog. 2019, 127, 70–78. [Google Scholar] [CrossRef]
- Patrzykat, A.; Friedrich, C.L.; Zhang, L.; Mendoza, V.; Hancock, R.E.W. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 2002, 46, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunologia 2003, 22, 277–286. [Google Scholar]
- Tafalla, C.; Leal, E.; Yamaguchi, T.; Fischer, U. T cell immunity in the teleost digestive tract. Dev. Comp. Immunol. 2016, 64, 167–177. [Google Scholar] [CrossRef]
- Ellis, A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Grinde, B.; Jollès, J.; Jollès, P. Purification and characterization of two lysozymes from rainbow trout (Salmo gairdneri). Eur. J. Biochem. 1988, 173, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Immunity to bacteria in fish. Fish Shellfish Immunol. 1999, 9, 291–308. [Google Scholar] [CrossRef]
- Watts, M.; Munday, B.L.; Burke, C.M. Immune responses of teleost fish. Aust. Vet. J. 2001, 79, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Shabir, U.; Ali, S.; Magray, A.R.; Ganai, B.A.; Firdous, P.; Hassan, T.; Nazir, R. Fish antimicrobial peptides (AMP’s) as essential and promising molecular therapeutic agents: A review. Microb. Pathog. 2018, 114, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Romany, J.S.; McElroy, A.E.; Fast, M.D. Measures of immune system status in young-of-the-year winter flounder Pseudopleuronectes americanus. J. Fish Biol. 2015, 86, 148–161. [Google Scholar] [CrossRef]
- Robohm, R.A.; Brown, C.; Murchelano, R.A. Comparison of antibodies in marine fish from clean and polluted waters of the New York Bight: Relative levels against 36 bacteria. Appl. Environ. Microbiol. 1979, 38, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Patrzykat, A.; Devlin, R.H.; Ackerman, P.A.; Iwama, G.K.; Hancock, R.E.W. Antimicrobial Peptides Protect Coho Salmon from Vibrio anguillarum Infections. Appl. Environ. Microbiol. 2000, 66, 1928–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, M.A.; Wolke, R.E.; Cabelli, V.J. Vibrio anguillarum as a cause of disease in winter flounder (Pseudopleuronectes americanus). Can. J. Microbiol. 1972, 18, 1585–1592. [Google Scholar] [CrossRef]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-F.; Du, Y.; Meng, L.; Li, X.; Yang, D.; Liu, Y. Phosphoproteomic analyses of kidneys of Atlantic salmon infected with Aeromonas salmonicida. Sci. Rep. 2019, 9, 2101. [Google Scholar] [CrossRef]
- Kim, R.; Faisal, M. Emergence and resurgence of the viral hemorrhagic septicemia virus (Novirhabdovirus, Rhabdoviridae, Mononegavirales). J. Adv. Res. 2011, 2, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Park, J.S.; Choi, M.C.; Kwon, S.R. Comparison of the efficacy of Poly(I:C) immunization with live vaccine and formalin-killed vaccine against viral hemorrhagic septicemia virus (VHSV) in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2016, 48, 206–211. [Google Scholar] [CrossRef]
- Douglas, S.E.; Patrzykat, A.; Pytyck, J.; Gallant, J.W. Identification, structure and differential expression of novel pleurocidins clustered on the genome of the winter flounder, Pseudopleuronectes americanus (Walbaum). Eur. J. Biochem. 2003, 270, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Patrzykat, A.; Gallant, J.W.; Seo, J.-K.; Pytyck, J.; Douglas, S.E. Novel Antimicrobial Peptides Derived from Flatfish Genes. Antimicrob. Agents Chemother. 2003, 47, 2464–2470. [Google Scholar] [CrossRef] [Green Version]
- Lauth, X.; Shike, H.; Burns, J.C.; Westerman, M.E.; Ostland, V.E.; Carlberg, J.M.; van Olst, J.C.; Nizet, V.; Taylor, S.W.; Shimizu, C.; et al. Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. J. Biol. Chem. 2002, 277, 5030–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, S.E.; Gallant, J.W.; Gong, Z.; Hew, C. Cloning and developmental expression of a family of pleurocidin-like antimicrobial peptides from winter flounder, Pleuronectes americanus (Walbaum). Dev. Comp. Immunol. 2001, 25, 137–147. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Jing, C.; Zhang, N.; Liang, D.; Xu, A. Identification, synthesis and characterization of a novel antimicrobial peptide HKPLP derived from Hippocampus kuda Bleeker. J. Antibiot. 2012, 65, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, A.M.; Darouiche, R.O.; Legarda, D.; Connell, N.; Diamond, G. Characterization of a Fish Antimicrobial Peptide: Gene Expression, Subcellular Localization, and Spectrum of Activity. Antimicrob. Agents Chemother. 2000, 44, 2039–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, H.M.; Gallant, J.W.; Douglas, S.E. Cellular localization of pleurocidin gene expression and synthesis in winter flounder gill using immunohistochemistry and in situ hybridization. Cell Tissue Res. 2003, 312, 197–202. [Google Scholar] [CrossRef]
- Mor, A.; Nguyen, V.H.; Delfour, A.; Migliore-Samour, D.; Nicolas, P. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 1991, 30, 8824–8830. [Google Scholar] [CrossRef] [PubMed]
- Marchini, D.; Manetti, A.G.O.; Rosetto, M.; Bernini, L.F.; Telford, J.L.; Baldari, C.T.; Dallai, R. cDNA Sequence and Expression of the Ceratotoxin Gene Encoding an Antibacterial Sex-specific Peptide from the Medfly Ceratitis capitata (diptera). J. Biol. Chem. 1995, 270, 6199–6204. [Google Scholar]
- Burrowes, O.J.; Hadjicharalambous, C.; Diamond, G.; Lee, T.-C. Evaluation of Antimicrobial Spectrum and Cytotoxic Activity of Pleurocidin for Food Applications. J. Food Sci. 2004, 69, FMS66–FMS71. [Google Scholar] [CrossRef]
- Tao, R.; Tong, Z.; Lin, Y.; Xue, Y.; Wang, W.; Kuang, R.; Wang, P.; Tian, Y.; Ni, L. Antimicrobial and antibiofilm activity of pleurocidin against cariogenic microorganisms. Peptides 2011, 32, 1748–1754. [Google Scholar] [CrossRef]
- Chou, H.-T.; Kuo, T.-Y.; Chiang, J.-C.; Pei, M.-J.; Yang, W.-T.; Yu, H.-C.; Lin, S.-B.; Chen, W.-J. Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. Int. J. Antimicrob. Agents 2008, 32, 130–138. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Chen, J.-Y.; Cheng, Y.-S.E.; Chen, C.-Y.; Ni, I.-H.; Sheen, J.-F.; Pan, Y.-L.; Kuo, C.-M. Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol. 2007, 26, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Mól, A.R.; Castro, M.S.; Fontes, W. NetWheels: A web application to create high quality peptide helical wheel and net projections. BioRxiv 2018, 416347. [Google Scholar] [CrossRef] [Green Version]
- Mason, A.J.; Chotimah, I.N.H.; Bertani, P.; Bechinger, B. A spectroscopic study of the membrane interaction of the antimicrobial peptide Pleurocidin. Mol. Membr. Biol. 2006, 23, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Talandashti, R.; Mahdiuni, H.; Jafari, M.; Mehrnejad, F. Molecular Basis for Membrane Selectivity of Antimicrobial Peptide Pleurocidin in the Presence of Different Eukaryotic and Prokaryotic Model Membranes. J. Chem. Inf. Model. 2019, 59, 3262–3276. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Hind, C.K.; Ferguson, P.M.; Amison, R.T.; Hodgson-Casson, A.C.; Ciazynska, K.A.; Weller, B.J.; Clarke, M.; Lam, C.; Man, R.C.H.; et al. A pleurocidin analogue with greater conformational flexibility, enhanced antimicrobial potency and in vivo therapeutic efficacy. Commun. Biol. 2020, 3, 697. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; He, P.; Xiao, C.; Chen, X. From Antimicrobial Peptides to Antimicrobial Poly(α-amino acid)s. Adv. Healthc. Mater. 2018, 7, 1800354. [Google Scholar] [CrossRef]
- Syvitski, R.T.; Burton, I.; Mattatall, N.R.; Douglas, S.E.; Jakeman, D.L. Structural characterization of the antimicrobial peptide pleurocidin from winter flounder. Biochemistry 2005, 44, 7282–7293. [Google Scholar] [CrossRef] [PubMed]
- Saint, N.; Cadiou, H.; Bessin, Y.; Molle, G. Antibacterial peptide pleurocidin forms ion channels in planar lipid bilayers. Biochim. Biophys. Acta 2002, 1564, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Lee, D.G. Oxidative stress by antimicrobial peptide pleurocidin triggers apoptosis in Candida albicans. Biochimie 2011, 93, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mukai, Y.; Niidome, T.; Takashi, C.; Tokunaga, Y.; Hatakeyama, T.; Aoyagi, H. Interaction of pleurocidin and its analogs with phospholipid membrane and their antibacterial activity. J. Pept. Res. 2001, 57, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. Pleurocidin-derived antifungal peptides with selective membrane-disruption effect. Biochem. Biophys. Res. Commun. 2008, 369, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Park, Y.; Sung, W.S.; Suh, B.K.; Lee, J.; Hahm, K.-S.; Lee, D.G. Fungicidal effect of pleurocidin by membrane-active mechanism and design of enantiomeric analogue for proteolytic resistance. Biochim. Biophys. Acta 2007, 1768, 1400–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilchie, A.L.; Haney, E.F.; Pinto, D.M.; Hancock, R.E.W.; Hoskin, D.W. Enhanced killing of breast cancer cells by a d-amino acid analog of the winter flounder-derived pleurocidin NRC-03. Exp. Mol. Pathol. 2015, 99, 426–434. [Google Scholar] [CrossRef]
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Wales, T.E.; Engen, J.R.; Walensky, L.D. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019, 37, 1186–1197. [Google Scholar] [CrossRef]
- Bryksa, B.C.; MacDonald, L.D.; Patrzykat, A.; Douglas, S.E.; Mattatall, N.R. A C-terminal glycine suppresses production of pleurocidin as a fusion peptide in Escherichia coli. Protein Expr. Purif. 2006, 45, 88–98. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.G. Antimicrobial peptide pleurocidin synergizes with antibiotics through hydroxyl radical formation and membrane damage, and exerts antibiofilm activity. Biochim. Biophys. Acta 2012, 1820, 1831–1838. [Google Scholar] [CrossRef]
- Riscal, R.; Skuli, N.; Simon, M.C. Even Cancer Cells Watch Their Cholesterol! Mol. Cell 2019, 76, 220–231. [Google Scholar] [CrossRef]
- Mai, J.; Tian, X.-L.; Gallant, J.W.; Merkley, N.; Biswas, Z.; Syvitski, R.; Douglas, S.E.; Ling, J.; Li, Y.-H. A novel target-specific, salt-resistant antimicrobial peptide against the cariogenic pathogen Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 5205–5213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wei, W.; Sun, Y.; Jiang, X.; Ying, X.; Tao, R.; Ni, L. Pleurocidin congeners demonstrate activity against Streptococcus and low toxicity on gingival fibroblasts. Arch. Oral Biol. 2016, 70, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Burrowes, O.-J.; Diamond, G.; Lee, T.-C. Recombinant Expression of Pleurocidin cDNA Using the Pichia pastoris Expression System. J. Biomed. Biotechnol. 2005, 2005, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Conrad, D.M.; Coombs, M.R.P.; Zemlak, T.; Doucette, C.D.; Liwski, R.S.; Hoskin, D.W. Pleurocidin-family cationic antimicrobial peptides mediate lysis of multiple myeloma cells and impair the growth of multiple myeloma xenografts. Leuk. Lymphoma 2013, 54, 2255–2262. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Doucette, C.D.; Pinto, D.M.; Patrzykat, A.; Douglas, S.; Hoskin, D.W. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 2011, 13, R102. [Google Scholar] [CrossRef] [Green Version]
- Morash, M.G.; Douglas, S.E.; Robotham, A.; Ridley, C.M.; Gallant, J.W.; Soanes, K.H. The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis. Model. Mech. 2011, 4, 622–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-Y.; Lin, W.-J.; Wu, J.-L.; Her, G.M.; Hui, C.-F. Epinecidin-1 peptide induces apoptosis which enhances antitumor effects in human leukemia U937 cells. Peptides 2009, 30, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Gill, E.E.; Coombs, M.R.P.; Falsafi, R.; Hancock, R.E.W.; Hoskin, D.W. MDA-MB-231 Breast Cancer Cells Resistant to Pleurocidin-Family Lytic Peptides Are Chemosensitive and Exhibit Reduced Tumor-Forming Capacity. Biomolecules 2020, 10, 1220. [Google Scholar] [CrossRef]
- Pundir, P.; Catalli, A.; Leggiadro, C.; Douglas, S.E.; Kulka, M. Pleurocidin, a novel antimicrobial peptide, induces human mast cell activation through the FPRL1 receptor. Mucosal Immunol. 2014, 7, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-C.; Huang, W.-L.; Kau, S.-W.; Yang, Y.-P.; Yang, C.-D. Pleurocidin Peptide Enhances Grouper Anti-Vibrio harveyi Immunity Elicited by Poly(lactide-co-glycolide)-Encapsulated Recombinant Glyceraldehyde-3-phosphate Dehydrogenase. Vaccines 2014, 2, 380–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.-P.; Chuang, S.-C.; Yang, C.-D. Protective Immunity against Vibrio harveyi in Grouper Induced by Single Vaccination with Poly (Lactide-co-glycolide) Microparticles Releasing Pleurocidin Peptide and Recombinant Glyceraldehyde-3-phosphate Dehydrogenase. Vaccines 2020, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, S.; Tafalla, C.; Cuesta, A.; Estepa, A.; Coll, J.M. In vitro search for alternative promoters to the human immediate early cytomegalovirus (IE-cMV) to express the G gene of viral haemorrhagic septicemia virus (VHSV) in fish epithelial cells. Vaccine 2008, 26, 6620–6629. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-J.; Kuo, T.-Y.; Lin, C.-C.; Chow, L.-P.; Chen, W.-J. Proteomic identification of membrane proteins regulating antimicrobial peptide resistance in Vibrio parahaemolyticus. J. Appl. Microbiol. 2010, 108, 1398–1407. [Google Scholar] [CrossRef]
- Dorrington, T.; Gomez-Chiarri, M. Antimicrobial Peptides for Use in Oyster Aquaculture: Effect on Pathogens, Commensals, and Eukaryotic Expression Systems. J. Shellfish Res. 2010, 27, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Eike, L.-M.; Yang, N.; Rekdal, Ø.; Sveinbjørnsson, B. The oncolytic peptide LTX-315 induces cell death and DAMP release by mitochondria distortion in human melanoma cells. Oncotarget 2015, 6, 34910–34923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacconelli, E.; Magrini, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]


| Potential Applications | Pleurocidin Characteristics | Reference(s) |
|---|---|---|
| Human | Low hemolysis | [37] |
| Induction of DNA fragmentation in cancer cells | [57] | |
| Upregulation of ROS in cancer cells and C. albicans | [44,58] | |
| Induction of mast cell granulation | [62] | |
| Cytotoxic against breast cancer cells | [48,58] | |
| Cytotoxic against myeloma cells | [57] | |
| Cytotoxic against leukemia cells | [60,61] | |
| Cytotoxic against MRSA | [31] | |
| Cytotoxic against S. mutans and S. sobrinus | [32,53,54] | |
| Effective against foodborne pathogens V. parahemolyticus, E. coli O157:H7, L. monocytogenes, S. cerevisiae, and P. expansum | [31] | |
| Aquaculture | Potential vaccine component against V. harveyi | [64] |
| Effective against oyster P. marinus | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMillan, K.A.M.; Coombs, M.R.P. Investigating Potential Applications of the Fish Anti-Microbial Peptide Pleurocidin: A Systematic Review. Pharmaceuticals 2021, 14, 687. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070687
McMillan KAM, Coombs MRP. Investigating Potential Applications of the Fish Anti-Microbial Peptide Pleurocidin: A Systematic Review. Pharmaceuticals. 2021; 14(7):687. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070687
Chicago/Turabian StyleMcMillan, Katelyn A. M., and Melanie R. Power Coombs. 2021. "Investigating Potential Applications of the Fish Anti-Microbial Peptide Pleurocidin: A Systematic Review" Pharmaceuticals 14, no. 7: 687. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070687