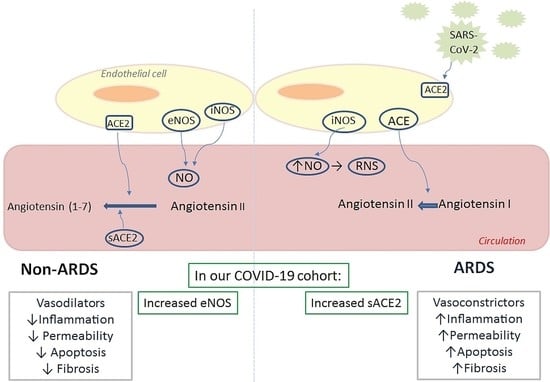
Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vane, J.R.; Anggard, E.E.; Botting, R.M. Regulatory functions of the vascular endothelium. N. Engl. J. Med. 1990, 323, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.D.; Evans, T.W. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur. Respir. J. 2003, 21, 720–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniatis, N.A.; Kotanidou, A.; Catravas, J.D.; Orfanos, S.E. Endothelial pathomechanisms in acute lung injury. Vasc. Pharmacol. 2008, 49, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef]
- Guan, S.P.; Seet, R.C.S.; Kennedy, B.K. Does eNOS derived nitric oxide protect the young from severe COVID–19 complications? Ageing Res. Rev. 2020, 64, 101201. [Google Scholar] [CrossRef]
- Abdih, H.; Kelly, C.J.; Bouchier–Hayes, D.; Watson, R.W.; Redmond, H.P.; Burke, P.; Bouchier–Hayes, D.J. Nitric oxide (endothelium–derived relaxing factor) attenuates revascularization–induced lung injury. J. Surg. Res. 1994, 57, 39–43. [Google Scholar] [CrossRef]
- Garrean, S.; Gao, X.P.; Brovkovych, V.; Shimizu, J.; Zhao, Y.Y.; Vogel, S.M.; Malik, A.B. Caveolin–1 regulates NF–kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol. 2006, 177, 4853–4860. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, A.; Pohl, C.B.; Sponholz, C.; Ma, N.; Stamm, C.; Vollmar, B.; Steinhoff, G. Up–regulation of endothelial nitric oxide synthase inhibits pulmonary leukocyte migration following lung ischemia–reperfusion in mice. Am. J. Pathol. 2004, 164, 2241–2249. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, A.; Kasch, C.; Zhang, L.; Kumar, S.; Sponholz, C.; Choi, Y.H.; Ma, N.; Liebold, A.; Ladilov, Y.; Steinhoff, G.; et al. Endothelial nitric oxide synthase mediates protective effects of hypoxic preconditioning in lungs. Respir. Physiol. Neurobiol. 2007, 155, 280–285. [Google Scholar] [CrossRef]
- Takenaka, K.; Nishimura, Y.; Nishiuma, T.; Sakashita, A.; Yamashita, T.; Kobayashi, K.; Satouchi, M.; Ishida, T.; Kawashima, S.; Yokoyama, M. Ventilator–induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1078–L1086. [Google Scholar] [CrossRef]
- Yamashita, T.; Kawashima, S.; Ohashi, Y.; Ozaki, M.; Ueyama, T.; Ishida, T.; Inoue, N.; Hirata, K.; Akita, H.; Yokoyama, M. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation 2000, 101, 931–937. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kumar, S.; Kaminski, A.; Kasch, C.; Sponholz, C.; Stamm, C.; Ladilov, Y.; Steinhoff, G. Importance of endothelial nitric oxide synthase for the hypothermic protection of lungs against ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 2006, 131, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Gielis, J.F.; Quirynen, L.; Briede, J.J.; Roelant, E.; Cos, P.; Van Schil, P.E.Y. Pathogenetic role of endothelial nitric oxide synthase uncoupling during lung ischaemia–reperfusion injury. Eur. J. Cardio Thorac. Surg. 2017, 52, 256–263. [Google Scholar] [CrossRef]
- Drucker, N.A.; Jensen, A.R.; Te Winkel, J.P.; Ferkowicz, M.J.; Markel, T.A. Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis. J. Pediatric Surg. 2018, 53, 1208–1214. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Gow, A.J.; Thom, S.R.; Ischiropoulos, H. Nitric oxide and peroxynitrite–mediated pulmonary cell death. Am. J. Physiol. 1998, 274, L112–L118. [Google Scholar] [CrossRef]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. Off. J. Soc. Toxicol. 2001, 59, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Müller, H.C.; Witzenrath, M.; Tschernig, T.; Gutbier, B.; Hippenstiel, S.; Santel, A.; Suttorp, N.; Rosseau, S. Adrenomedullin attenuates ventilator–induced lung injury in mice. Thorax 2010, 65, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Obata, H.; Murakami, S.; Hamada, K.; Kangawa, K.; Kimura, H.; Nagaya, N. Adrenomedullin ameliorates lipopolysaccharide–induced acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L446–L452. [Google Scholar] [CrossRef]
- Pagliaro, P.; Thairi, C.; Alloatti, G.; Penna, C. Angiotensin–converting enzyme 2: A key enzyme in key organs. J. Cardiovasc. Med. 2021. [Google Scholar] [CrossRef]
- Orfanos, S.E.; Armaganidis, A.; Glynos, C.; Psevdi, E.; Kaltsas, P.; Sarafidou, P.; Catravas, J.D.; Dafni, U.G.; Langleben, D.; Roussos, C. Pulmonary capillary endothelium–bound angiotensin–converting enzyme activity in acute lung injury. Circulation 2000, 102, 2011–2018. [Google Scholar] [CrossRef] [Green Version]
- Kraaijvanger, R.; Janssen Bonás, M.; Vorselaars, A.D.M.; Veltkamp, M. Biomarkers in the Diagnosis and Prognosis of Sarcoidosis: Current Use and Future Prospects. Front. Immunol. 2020, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID–19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Wood, K.C.; Cortese–Krott, M.M.; Kovacic, J.C.; Noguchi, A.; Liu, V.B.; Wang, X.; Raghavachari, N.; Boehm, M.; Kato, G.J.; Kelm, M.; et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1861–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotz, C.; Muellenbach, R.M.; Meybohm, P.; Mutlak, H.; Lepper, P.M.; Rolfes, C.B.; Peivandi, A.; Stumpner, J.; Kredel, M.; Kranke, P.; et al. Effects of inhaled nitric oxide in COVID–19–induced ARDS–Is it worthwhile? Acta Anaesthesiol. Scand. 2021, 65, 629–632. [Google Scholar] [CrossRef]
- Longobardo, A.; Montanari, C.; Shulman, R.; Benhalim, S.; Singer, M.; Arulkumaran, N. Inhaled nitric oxide minimally improves oxygenation in COVID–19 related acute respiratory distress syndrome. Br. J. Anaesth. 2021, 126, e44–e46. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pozzi, M.; Mongodi, S.; Dammassa, V.; Romito, G.; Mojoli, F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID–19 pneumonia. Crit. Care 2020, 24, 508. [Google Scholar] [CrossRef]
- Ferrari, M.; Santini, A.; Protti, A.; Andreis, D.T.; Iapichino, G.; Castellani, G.; Rendiniello, V.; Costantini, E.; Cecconi, M. Inhaled nitric oxide in mechanically ventilated patients with COVID–19. J. Crit. Care 2020, 60, 159–160. [Google Scholar] [CrossRef]
- Hupf, J.; Mustroph, J.; Hanses, F.; Evert, K.; Maier, L.S.; Jungbauer, C.G. RNA–expression of adrenomedullin is increased in patients with severe COVID–19. Crit. Care 2020, 24, 527. [Google Scholar] [CrossRef]
- Wilson, D.C.; Schefold, J.C.; Baldirà, J.; Spinetti, T.; Saeed, K.; Elke, G. Adrenomedullin in COVID–19 induced endotheliitis. Crit. Care 2020, 24, 411. [Google Scholar] [CrossRef]
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Lockey, R.F.; Kolliputi, N. Soluble ACE2 as a potential therapy for COVID–19. Am. J. Physiol. Cell Physiol. 2021, 320, C279–C281. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B., Jr.; Fejes, Z.; Szentkereszty, Z.; Sütő, R.; Várkonyi, I.; Ajzner, É.; Kappelmayer, J.; Papp, Z.; Tóth, A.; Fagyas, M. A dramatic rise in serum ACE2 activity in a critically ill COVID–19 patient. Int. J. Infect. Dis. 2021, 103, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hasan, M.; Ahmed, A. Potential detrimental role of soluble ACE2 in severe COVID–19 comorbid patients. Rev. Med Virol. 2021. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Trusa, S.; Olson, S.C. Angiotensin type 1 receptor is linked to inhibition of nitric oxide production in pulmonary endothelial cells. Regul. Pept. 2005, 132, 113–122. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. The potential role of serum angiotensin–converting enzyme in coronavirus disease 2019. BMC Infect. Dis. 2020, 20, 883. [Google Scholar] [CrossRef]
- Avanoglu Guler, A.; Tombul, N.; Aysert Yıldız, P.; Özger, H.S.; Hızel, K.; Gulbahar, O.; Tufan, A.; Erbaş, G.; Aygencel, G.; Guzel Tunçcan, O.; et al. The assessment of serum ACE activity in COVID–19 and its association with clinical features and severity of the disease. Scand. J. Clin. Lab. Investig. 2021, 81, 160–165. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]

| Characteristics | ARDS | Non-ARDS | P-value | Reference Values |
|---|---|---|---|---|
| Number of patients, N | 68 | 21 | ||
| Age (years), (mean ± SD) | 62 ± 13 | 61 ± 13 | 0.8 | |
| Sex, N (%) | >0.9 | |||
| Male | 53 (77.9) | 17 (80.9) | ||
| Female | 15 (22.1) | 4 (19.1) | ||
| Comorbidities, N (%) | 50 (73.5) | 17 (80.9) | 0.6 | |
| ICU vs. ward, N (%) | <0.0001 * | |||
| ICU | 60 (88.2) | 7 (33.3) | ||
| Ward | 8 (11.8) | 14 (66.7) | ||
| Sick days prior to admission, (mean ± SD) | 7 ± 3 | 6 ± 4 | 0.2 | |
| APACHE II, (mean ± SD) † | 14 ± 5 | 8 ± 6 | <0.0001 * | |
| SOFA, (median, IQR) † | 6 (4–8) | 3 (2–3) | <0.0001 * | |
| White blood cell count (per μL), (median, IQR) Neutrophils (%), (median, IQR) Lymphocytes (%), (median, IQR) Platelets (per μL), (median, IQR) CRP (mg/dL), (median, IQR) Fibrinogen (mg/dL), (mean ± SD) D-dimers (µg/mL), (median, IQR) LDH (U/L), (median, IQR) Ferritin (ng/mL), (median, IQR) Lactate (mmol/L), (mean ± SD) | 8760 (5915–11,370) 83 (76–88) 12 (7–17) 225,000 (169,000–27,000) 11.5 (5.4–20.4) 630 ± 179 1.05 (0.46–2.29) 434 (339–574) 682 (265–2006) 1.5 ± 0.6 | 5630 (4355–12,405) 83 (63–87) 13 (9–29) 195,000 (155,250–269,500) 4.8 (1.6–11.1) 548 ± 151 0.74 (0.51–1.36) 257 (211–391) 376 (164–868) 1.4 ± 0.5 | 0.2 0.6 0.2 0.3 0.002 * 0.1 0.2 <0.0001 * 0.2 0.8 | 4–10.5 × 103 40–70 25–45 140–450 × 103 <0.5 200–400 <0.5 <225 10–250 <2.0 |
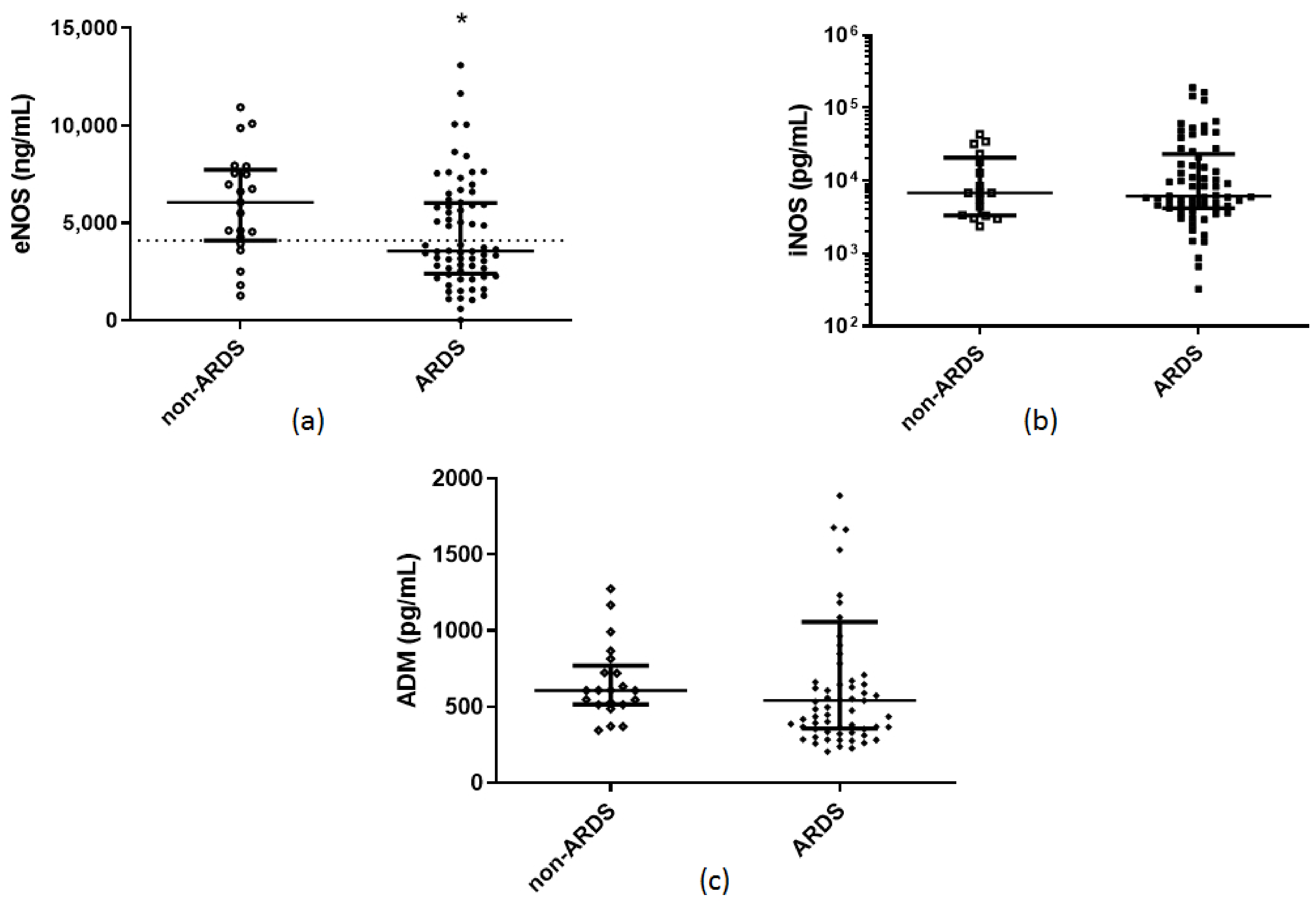
| Soluble endothelial–related molecules eNOS (ng/mL), (median, IQR) iNOS (pg/mL), (median, IQR) ADM (pg/mL), (median, IQR) sACE2 (pg/mL), (median, IQR) sACE (U/L), (median, IQR) | 3570 (2405–6030) 6138 (4200–23,131) 543 (357–1057) 8700 (4313–13,325) 30 (23–43) | 6070 (4025–7830) 6763 (3300–23,288) 608 (514–794) 125 (10–5225) 29 (18–42) | 0.02 * 0.7 0.4 <0.0001 * 0.5 | |
| Outcomes | ||||
| Mechanical ventilation, N (%) | 50 (73.5) | 4 (19.0) | <0.0001 * | |
| MV duration (days), (median, IQR) | 8 (1–22) | 0 (0–0) | <0.001 * | |
| LoS (days), (median, IQR) | 18 (13–30) | 8 (6–22) | 0.002 * | |
| In–hospital mortality, N (%) | 23 (33.8) | 3 (14.3) | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassiliou, A.G.; Zacharis, A.; Keskinidou, C.; Jahaj, E.; Pratikaki, M.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals 2021, 14, 695. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070695
Vassiliou AG, Zacharis A, Keskinidou C, Jahaj E, Pratikaki M, Gallos P, Dimopoulou I, Kotanidou A, Orfanos SE. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals. 2021; 14(7):695. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070695
Chicago/Turabian StyleVassiliou, Alice G., Alexandros Zacharis, Chrysi Keskinidou, Edison Jahaj, Maria Pratikaki, Parisis Gallos, Ioanna Dimopoulou, Anastasia Kotanidou, and Stylianos E. Orfanos. 2021. "Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS)" Pharmaceuticals 14, no. 7: 695. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14070695