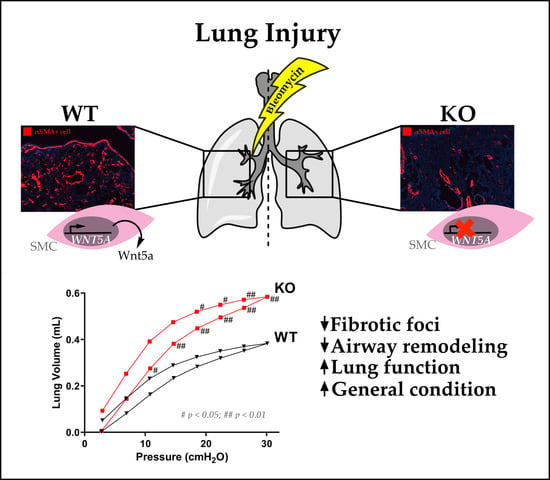
The Pathogenic Role of Smooth Muscle Cell-Derived Wnt5a in a Murine Model of Lung Fibrosis
Abstract
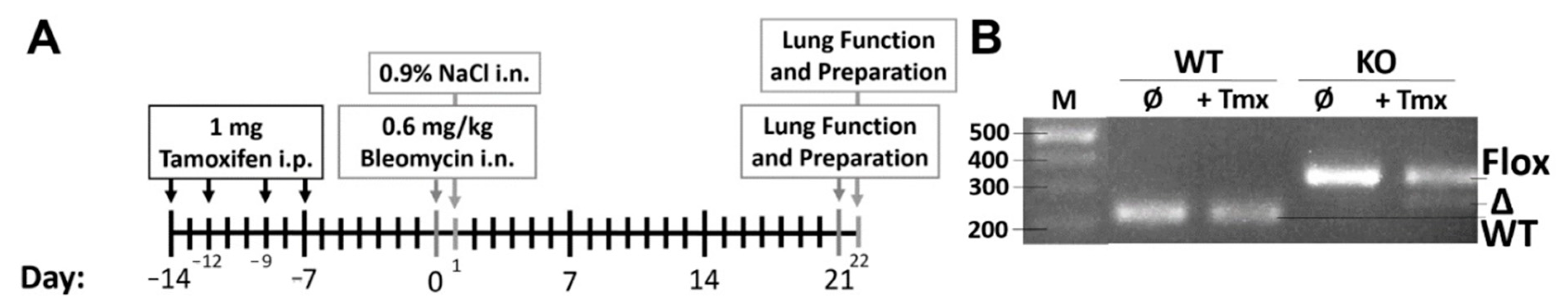
:1. Introduction
2. Results
2.1. Airway Smooth Muscle-Derived Wnt5a Contributes to Worse Clinical Symptoms and Lung Function
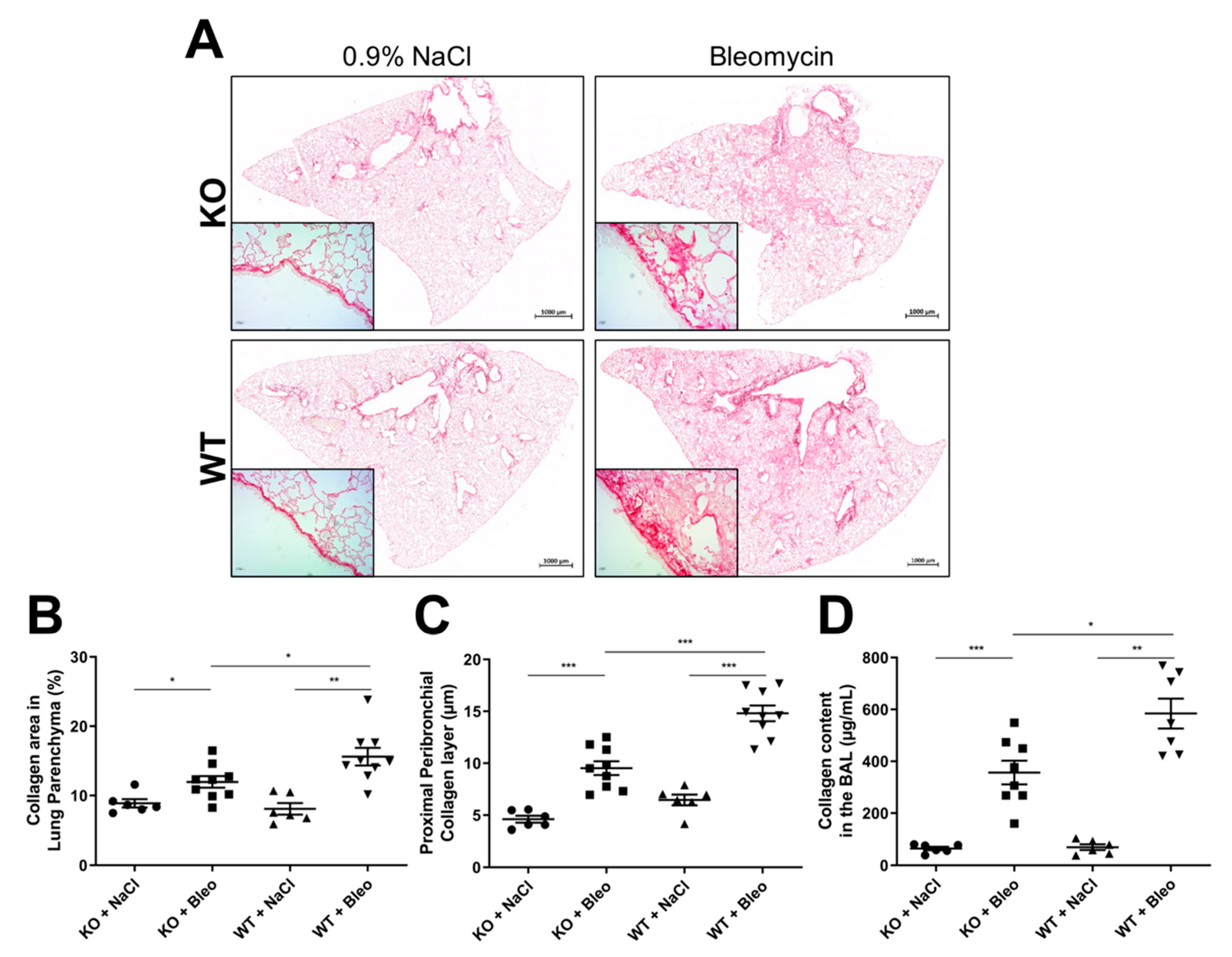
2.2. Loss of Wnt5a Expression in Airway Smooth Muscle Cells Ameliorates Fibrogenesis
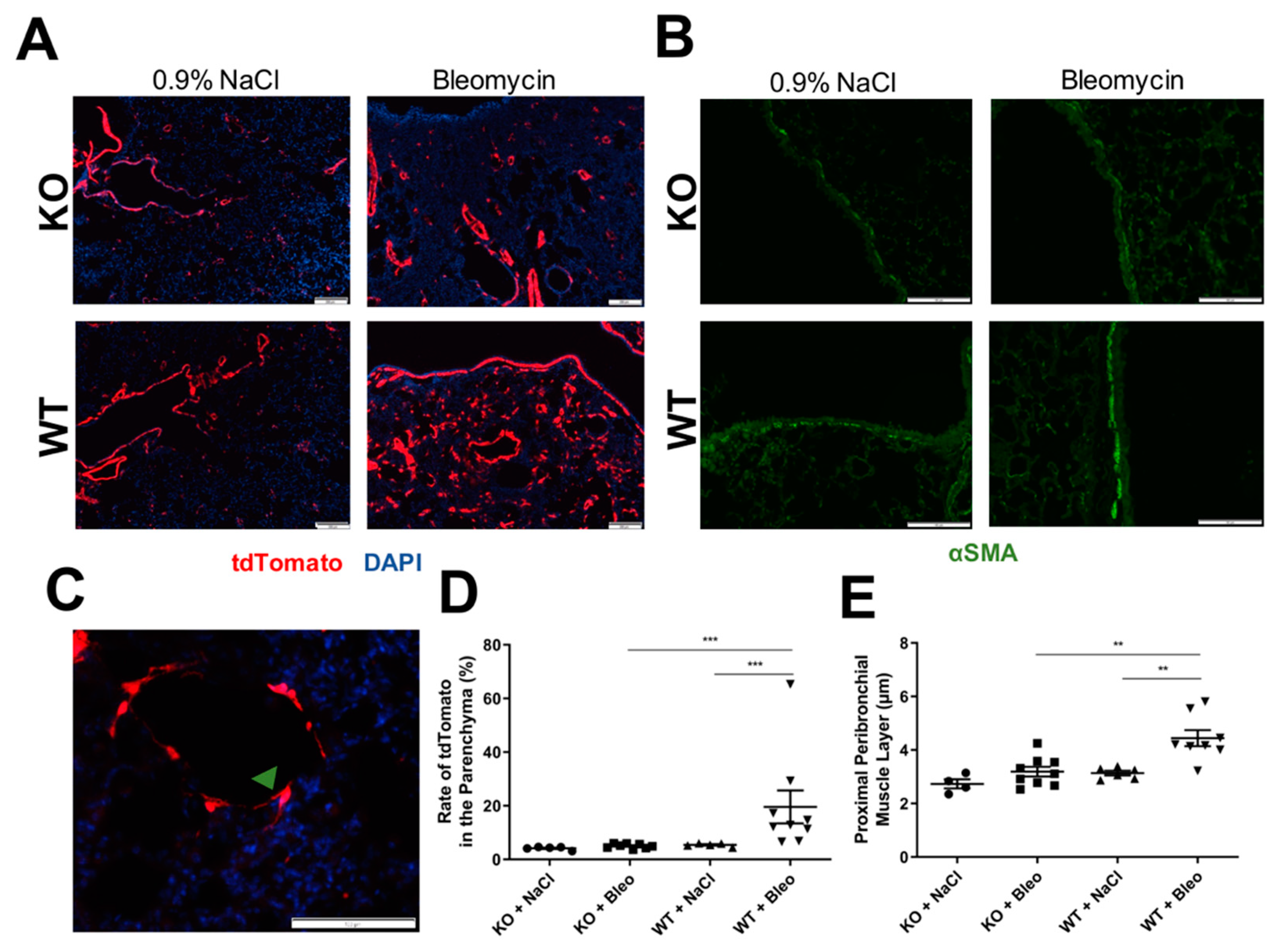
2.3. Airway Smooth Muscle Contributes to Asma+ Cell Accumulation in the Parenchyma and to Its Own Hyperplasia through Wnt5a Expression
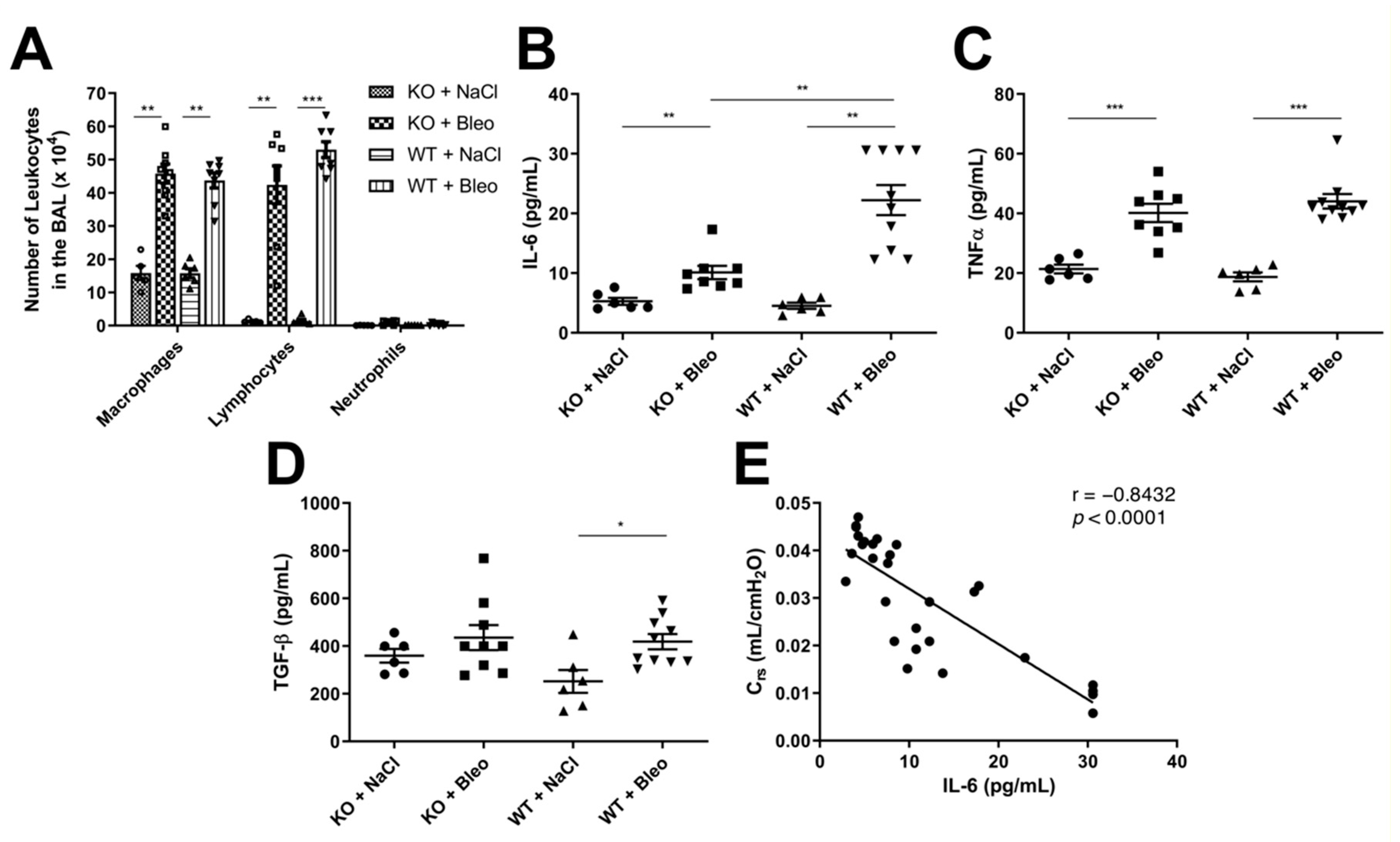
2.4. Deletion of the Wnt5a Gene in Airway Smooth Muscle Cells Does Not Affect Leukocyte Infiltration into the Airways but Has Mixed Effects on Cytokine Expression
3. Discussion
4. Materials and Methods
4.1. Animal Ethical Approval
4.2. Mice
4.3. Bleomycin-Induced Fibrosis
4.4. Characterization of Wnt5a Knockout
4.5. Lung Function
4.6. Lung Samples and Analysis
4.7. Histological Analysis
4.8. Immunofluorescence Staining and Detection
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.R.; Sills, W.S.; Hanrahan, K.; Ziegler, A.; Tidd, K.M.; Cook, E.; Sannes, P.L. Expression of WNT5A in Idiopathic Pulmonary Fibrosis and Its Control by TGF-β and WNT7B in Human Lung Fibroblasts. J. Histochem. Cytochem. 2016, 64, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Loscertales, M.; Mikels, A.J.; Hu, J.K.-H.; Donahoe, P.K.; Roberts, D.J. Chick Pulmonary Wnt5a Directs Airway and Vascular Tubulogenesis. Development 2008, 135, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Beljaars, L.; Daliri, S.; Dijkhuizen, C.; Poelstra, K.; Gosens, R. WNT-5A Regulates TGF-β-Related Activities in Liver Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G219–G227. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Liang, Y.; Zhu, X.; Wang, M.; Gui, Y.; Lu, Q.; Gu, M.; Xue, X.; Sun, X.; He, W.; et al. The Signaling Protein Wnt5a Promotes TGFβ1-Mediated Macrophage Polarization and Kidney Fibrosis by Inducing the Transcriptional Regulators Yap/Taz. J. Biol. Chem. 2018, 293, 19290–19302. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.Y.; Park, H.H.; Kim, H.; Kim, H.N.; Kim, I.; Jeon, S.; Kim, W.; Bae, J.-S.; Lee, W. Wnt5a and Wnt11 as Acute Respiratory Distress Syndrome Biomarkers for Severe Acute Respiratory Syndrome Coronavirus 2 Patients. Eur. Respir. J. 2020, 56, 2001531. [Google Scholar] [CrossRef]
- Seifert, J.R.K.; Mlodzik, M. Frizzled/PCP Signalling: A Conserved Mechanism Regulating Cell Polarity and Directed Motility. Nat. Rev. Genet. 2007, 8, 126–138. [Google Scholar] [CrossRef]
- Carvajal-Gonzalez, J.M.; Balmer, S.; Mendoza, M.; Dussert, A.; Collu, G.; Roman, A.-C.; Weber, U.; Ciruna, B.; Mlodzik, M. The Clathrin Adaptor AP-1 Complex and Arf1 Regulate Planar Cell Polarity in Vivo. Nat. Commun. 2015, 6, 6751. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, M.; Smith, K.; Harris, A.L. Regulation of Wnt5a Expression in Human Mammary Cells by Protein Kinase C Activity and the Cytoskeleton. Br. J. Cancer 1998, 78, 430–438. [Google Scholar] [CrossRef]
- Saneyoshi, T.; Kume, S.; Amasaki, Y.; Mikoshiba, K. The Wnt/Calcium Pathway Activates NF-AT and Promotes Ventral Cell Fate in Xenopus Embryos. Nature 2002, 417, 295–299. [Google Scholar] [CrossRef]
- Macian, F. NFAT Proteins: Key Regulators of T-Cell Development and Function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a Protein Activates or Inhibits Beta-Catenin-TCF Signaling Depending on Receptor Context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Mutze, K.; Baarsma, H.A.; Bracke, K.R.; Alsafadi, H.N.; Lehmann, M.; Costa, R.; Stornaiuolo, M.; Novellino, E.; Brusselle, G.G.; et al. Reduced Frizzled Receptor 4 Expression Prevents WNT/β-Catenin-Driven Alveolar Lung Repair in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 172–185. [Google Scholar] [CrossRef]
- Huang, C.; Xiao, X.; Yang, Y.; Mishra, A.; Liang, Y.; Zeng, X.; Yang, X.; Xu, D.; Blackburn, M.R.; Henke, C.A.; et al. MicroRNA-101 Attenuates Pulmonary Fibrosis by Inhibiting Fibroblast Proliferation and Activation. J. Biol. Chem. 2017, 292, 16420–16439. [Google Scholar] [CrossRef] [Green Version]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.A.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.; Chen, G.; Stephens, N.; Hirst, S.; Douglas, D.; Eichholtz, T.; Khalil, N. Release of Biologically Active TGF-Beta from Airway Smooth Muscle Cells Induces Autocrine Synthesis of Collagen. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2001, 280, L999–L1008. [Google Scholar] [CrossRef]
- Horsnell, W.G.C.; Vira, A.; Kirstein, F.; Mearns, H.; Hoving, J.C.; Cutler, A.J.; Dewals, B.; Myburgh, E.; Kimberg, M.; Arendse, B.; et al. IL-4Rα-Responsive Smooth Muscle Cells Contribute to Initiation of TH2 Immunity and Pulmonary Pathology in Nippostrongylus Brasiliensis Infections. Mucosal Immunol. 2011, 4, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.; Menzen, M.H.; Bos, I.S.T.; Baarsma, H.A.; Borger, P.; Roth, M.; Tamm, M.; Halayko, A.J.; Simoons, M.; Prins, A.; et al. Noncanonical WNT-5A Signaling Regulates TGF-β-Induced Extracellular Matrix Production by Airway Smooth Muscle Cells. FASEB J. 2013, 27, 1631–1643. [Google Scholar] [CrossRef]
- Sausville, E.A.; Stein, R.W.; Peisach, J.; Horwitz, S.B. Properties and Products of the Degradation of DNA by Bleomycin and Iron(II). Biochemistry 1978, 17, 2746–2754. [Google Scholar] [CrossRef]
- Sleijfer, S. Bleomycin-Induced Pneumonitis. Chest 2001, 120, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wan, J.; Han, J.-Z.; Li, C.; Feng, D.-D.; Yue, S.-J.; Huang, Y.-H.; Chen, Y.; Cheng, Q.-M.; Li, Y.; et al. Antiflammin-1 Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice. Respir. Res. 2013, 14, 101. [Google Scholar] [CrossRef] [Green Version]
- De Wever, O.; Westbroek, W.; Verloes, A.; Bloemen, N.; Bracke, M.; Gespach, C.; Bruyneel, E.; Mareel, M. Critical Role of N-Cadherin in Myofibroblast Invasion and Migration in Vitro Stimulated by Colon-Cancer-Cell-Derived TGF-Beta or Wounding. J. Cell Sci. 2004, 117, 4691–4703. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, C.-L.; Li, R.-M.; Hui, T.-Q.; Su, Y.-Y.; Yuan, Q.; Zhou, X.-D.; Ye, L. Wnt5a Promotes Inflammatory Responses via Nuclear Factor ΚB (NF-ΚB) and Mitogen-Activated Protein Kinase (MAPK) Pathways in Human Dental Pulp Cells. J. Biol. Chem. 2014, 289, 21028–21039. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Fu, J.; Liu, X.; Xue, X. ERK1/2 Signaling Pathway Activated by EGF Promotes Proliferation, Transdifferentiation, and Migration of Cultured Primary Newborn Rat Lung Fibroblasts. Biomed Res. Int. 2020, 2020, 7176169. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Bahri, S.; Ben Ali, R.; Nahdi, A.; Mlika, M.; Abdennabi, R.; Jameleddine, S. Salvia Officinalis Attenuates Bleomycin-Induced Oxidative Stress and Lung Fibrosis in Rats. Nutr. Cancer 2020, 72, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Smith, S.M.; Peinado, N.; Gao, F.; Li, W.; Lee, M.K.; Zhou, B.; Bellusci, S.; Pryhuber, G.S.; Ho, H.-Y.H.; et al. WNT5a-ROR Signaling Is Essential for Alveologenesis. Cells 2020, 9, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampetaki, A.; Zhang, Z.; Hu, Y.; Xu, Q. Biomechanical Stress Induces IL-6 Expression in Smooth Muscle Cells via Ras/Rac1-P38 MAPK-NF-KappaB Signaling Pathways. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2946–H2954. [Google Scholar] [CrossRef]
- Veldhuizen, R.A.; Slutsky, A.S.; Joseph, M.; McCaig, L. Effects of Mechanical Ventilation of Isolated Mouse Lungs on Surfactant and Inflammatory Cytokines. Eur. Respir. J. 2001, 17, 488–494. [Google Scholar] [CrossRef]
- Barbero, G.; Castro, M.V.; Villanueva, M.B.; Quezada, M.J.; Fernández, N.B.; DeMorrow, S.; Lopez-Bergami, P. An Autocrine Wnt5a Loop Promotes NF-ΚB Pathway Activation and Cytokine/Chemokine Secretion in Melanoma. Cells 2019, 8, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, S.; Zelazny, E.T.; Souhrada, J.F.; Souhrada, M. IL-1 Beta and IL-6 Induce Hyperplasia and Hypertrophy of Cultured Guinea Pig Airway Smooth Muscle Cells. J. Appl. Physiol. 1995, 78, 1555–1563. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in Asthma and Other Inflammatory Pulmonary Diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Koopmans, T.; Hesse, L.; Nawijn, M.C.; Kumawat, K.; Menzen, M.H.; Bos, I.S.T.; Smits, R.; Bakker, E.R.M.; van den Berge, M.; Koppelman, G.H.; et al. Smooth-Muscle-Derived WNT5A Augments Allergen-Induced Airway Remodelling and Th2 Type Inflammation. Sci. Rep. 2020, 10, 6754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, J.M.; Goetz, M.P.; Buhrow, S.A.; Walden, C.; Safgren, S.L.; Kuffel, M.J.; Reinicke, K.E.; Suman, V.; Haluska, P.; Hou, X.; et al. Pharmacokinetics of Endoxifen and Tamoxifen in Female Mice: Implications for Comparative in Vivo Activity Studies. Cancer Chemother. Pharmacol. 2014, 74, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Zhang, C.; Zhang, Q.; Miele, L.; Zheng, S.; Wang, G. Boronic Prodrug of 4-Hydroxytamoxifen Is More Efficacious than Tamoxifen with Enhanced Bioavailability Independent of CYP2D6 Status. BMC Cancer 2015, 15, 625. [Google Scholar] [CrossRef] [Green Version]
- Wendling, O.; Bornert, J.-M.; Chambon, P.; Metzger, D. Efficient Temporally-Controlled Targeted Mutagenesis in Smooth Muscle Cells of the Adult Mouse. Genesis 2009, 47, 14–18. [Google Scholar] [CrossRef]
- Miyoshi, H.; Ajima, R.; Luo, C.T.; Yamaguchi, T.P.; Stappenbeck, T.S. Wnt5a Potentiates TGF-β Signaling to Promote Colonic Crypt Regeneration after Tissue Injury. Science 2012, 338, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devos, F.C.; Maaske, A.; Robichaud, A.; Pollaris, L.; Seys, S.; Lopez, C.A.; Verbeken, E.; Tenbusch, M.; Lories, R.; Nemery, B.; et al. Forced Expiration Measurements in Mouse Models of Obstructive and Restrictive Lung Diseases. Respir. Res. 2017, 18, 123. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmo-Fernandes, A.; Puschkarow, M.; Peters, K.; Gnipp, S.; Peters, M. The Pathogenic Role of Smooth Muscle Cell-Derived Wnt5a in a Murine Model of Lung Fibrosis. Pharmaceuticals 2021, 14, 755. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14080755
Carmo-Fernandes A, Puschkarow M, Peters K, Gnipp S, Peters M. The Pathogenic Role of Smooth Muscle Cell-Derived Wnt5a in a Murine Model of Lung Fibrosis. Pharmaceuticals. 2021; 14(8):755. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14080755
Chicago/Turabian StyleCarmo-Fernandes, André, Michelle Puschkarow, Karin Peters, Stefanie Gnipp, and Marcus Peters. 2021. "The Pathogenic Role of Smooth Muscle Cell-Derived Wnt5a in a Murine Model of Lung Fibrosis" Pharmaceuticals 14, no. 8: 755. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14080755