Formulation and Evaluation of Helichrysum italicum Essential Oil-Based Topical Formulations for Wound Healing in Diabetic Rats
Abstract
:1. Introduction
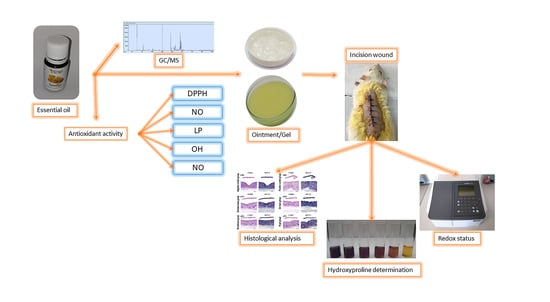
2. Results
2.1. GC-MS Analysis of H. italicum Essential Oil
2.2. Antioxidant Activity
2.3. Percentage Wound Contraction
2.4. Hydroxyproline Content
2.5. Redox Status
2.6. Histologic Analysis
3. Discussion
4. Materials and Methods
4.1. Test Compounds
4.2. Phytochemical Analysis of H. italicum Essential Oil
4.3. Antioxidant Potential Evaluation
4.4. Preparation of the Test Formulations
4.5. In Vivo Wound Healing Studies
4.5.1. Animals
4.5.2. Induction of Diabetes
4.5.3. Incision Wound Model
4.5.4. Experimental Animals
- Negative control, the wound was left without intervention
- Positive control (the wound was treated with 1% silver sulfadiazine)
- Ointment base (the wound was treated with Eucerin base ointment)
- Gel base (the wound was treated with Carbomer mucilago gel)
- HIEO ointment (the wound was treated with the 0.5% H. italicum essential oil ointment).
- HIEO gel (the wound was treated with the 0.5% H. italicum essential oil gel).
4.5.5. Estimation of Wound Contraction and Epithelialization Period
4.6. Biochemical Analysis
4.6.1. Hydroxyproline Estimation
4.6.2. Evaluation of Systemic Redox State
Determination of the Index of Lipid Peroxidation Measured as TBARS
Nitrite Determination (NO2−)
Superoxide Anion Radical Determination (O2−)
Hydrogen Peroxide Determination (H2O2)
Determination of Reduced Glutathione (GSH)
Determination of Catalase (CAT)
Determination of Superoxide Dismutase (SOD)
4.7. Histologic Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.; Zhao, C.; Yang, L.; Qi, X.; Wang, X.; Zhou, Q.; Shi, W. Hyperglycemia-reduced NAD+ biosynthesis impairs corneal epithelial wound healing in diabetic mice. Metabolism 2021, 114, 154402. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Turk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Hashemnia, M.; Nikousefat, Z.; Mohammadalipour, A.; Zangeneh, M.M.; Zangeneh, A. Wound healing activity of Pimpinella anisum methanolic extract in streptozotocin-induced diabetic rats. J. Wound Care 2019, 28, S26–S36. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.S.; Heo, C.Y.; Shin, J.; Moon, S.Y.; Cho, S.W.; Kang, H.J. Effects of a catechol-functionalized hyaluronic acid patch combined with human adipose-derived stem cells in diabetic wound healing. Int. J. Mol. Sci. 2021, 22, 2632. [Google Scholar] [CrossRef] [PubMed]
- Toygar, I.; Tureyen, A.; Demir, D.; Cetinkalp, S. Effect of allicin on wound healing: An experimental diabetes model. J. Wound Care 2020, 29, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Ambryszewska, K.E.; Melai, B.; Flamini, G.; Cioni, P.L.; Parri, F.; Pistelli, L. Essential oil composition of H. italicum (Roth) G. Don ssp. italicum from Elba Island (Tuscany, Italy). Chem Biodivers. 2013, 10, 343–355. [Google Scholar] [CrossRef]
- Kladar, N.V.; Anačkov, G.T.; Rat, M.M.; Srđenović, B.U.; Grujić, N.N.; Šefer, E.I.; Božin, B.N. Biochemical characterization of H. italicum (Roth) G. Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy, and antioxidant properties. Chem. Biodivers. 2015, 12, 419–431. [Google Scholar] [CrossRef]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Tadić, V.; Costantini, S.; Ragno, R.; Božović, M. Chemical composition and antimicrobial activity of essential oil of H. italicum (Roth) G. Don fil. (Asteraceae) from Montenegro. Nat. Prod. Res. 2019, 34, 445–448. [Google Scholar] [CrossRef]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, H.J.; Maria, A.E.; Mohamed Mihoub, Z. Chemical constituents of H. italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2017, 25, 780–787. [Google Scholar] [CrossRef]
- Mastelić, J.; Politeo, O.; Jerković, I. Contribution to the analysis of the essential oil of H. italicum (Roth) G. Don.—Determination of Ester Bonded Acids and Phenols. Molecules 2018, 13, 795. [Google Scholar] [CrossRef] [Green Version]
- Mancini, E.; De Martino, L.; Marandino, A.; Scognamiglio, M.R.; De Feo, V. Chemical composition and possible in vitro phytotoxic activity of Helichrsyum italicum (Roth) Don ssp. italicum. Molecules 2011, 16, 7725. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbeka, H.; Guvenalpa, Z. In vivo bioactivity assessment on Epilobium species: A particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Medić-Zamaklar, M. Diabetes Mellitus; Elit Medica: Beograd, Serbia, 1997; pp. 1–14. [Google Scholar]
- Blažević, N.; Petričić, J.; Stanić, G.; Maleš, Ž. Variations in yields and composition of immortelle (H. italicum, Roth Guss.) essential oil from different locations and vegetation periods along Adriatic coast. Acta Pharmaceutica 1995, 45, 517–522. [Google Scholar]
- Mastelic, J.; Politeo, O.; Jerkovic, I.; Radosevic, N. Composition and antimicrobial activity of H. italicum essential oil and its terpene and terpenoids fractions. Chem. Nat. Compd. 2005, 41, 35–39. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V.H. italicum (Roth) G. Don essential oil from serbia: Chemical composition, classification and biological activity—May it be a suitable new crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Bianchini, A.; Tomi, P.; Bernardini, A.F.; Morelli, I.; Flamini, G.; Cioni, P.L.; Usaï, M.; Marchetti, M. A comparative study of volatile constituents of two H. italicum (Roth) Guss. Don Fil subspecies growing in Corsica (France), Tuscany and Sardinia (Italy). Flavour Fragr. J. 2003, 18, 487–491. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Petrakis, P.V.; Chinou, I.; Skoula, M.; Harborne, J.B. Volatile, constituents of four Helichrysum species growing in Greece. Biochem. Syst. Ecol. 2000, 28, 163–175. [Google Scholar] [CrossRef]
- Chinou, I.B.; Roussis, V.; Perdetzoglou, D.; Loukis, A. Chemical and biological studies of two Helichrysum species of Greek origin. Planta Med. 1996, 62, 377–379. [Google Scholar] [CrossRef]
- Mollova, S.; Fidan, H.; Antonova, D.; Bozhilov, D.; Stanev, S.; Kostova, I.; Stoyanova, A. Chemical composition and antimicrobial and antioxidant activity of H. italicum (Roth) G.Don subspecies essential oils. Turk. J. Agric. For. 2020, 44, 371–378. [Google Scholar] [CrossRef]
- Emami, S.A.; Javadi, B.; Hassanzadeh, M.K. Antioxidant activity of the essential oils of different parts of Juniperus communis. subsp. hemisphaerica. and Juniperus oblonga. Pharm. Biol. 2007, 10, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Dzamic, A.M.; Mileski, K.S.; Ciric, A.D.; Ristic, M.S.; Sokovic, M.D.; Marin, P.D. Essential oil composition, antioxidant and antimicrobial properties of essential oil and deodorized extracts of H. italicum (Roth) G. Don. J. Essent. Oil Bear. Plants 2019, 22, 493–503. [Google Scholar] [CrossRef]
- Han, X.; Beaumont, C.; Stevens, N. Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cheng, H.L.; Kuan, Y.H.; Liang, T.J.; Chao, Y.Y.; Lin, H.C. Therapeutic potential of luteolin on impaired wound healing in streptozotocin-induced rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Xu, J.; Tian, Y.; Liu, W.; Peng, B. Marine fish peptides (collagen peptides) compound intake promotes wound healing in rats after cesarean section. J. Food Nutr. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- Ashja Zadeh, M.A.; Ebrahimi, M.; Salarian, A.A.; Abtahi, S.R.; Jahandideh, A. Evaluation of beneficial influence of local application of Crocus Pallasii Subsp. Haussknechtii Boiss. Extract on healing of full thickness excisional infected wounds in diabetic rats. Bull. Emer. Trauma 2020, 8, 169–178. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P.; Verma, A. In silico study of Trianthema portulacastrum embedded iron oxide nanoparticles on glycogen synthase kinase-3β: A possible contributor to its enhanced in vivo wound healing potential. Front. Pharmacol. 2021, 12, 664075. [Google Scholar] [CrossRef] [PubMed]
- Ponrasu, T.; Jamuna, S.; Mathew, A.; Madhukumar, K.N.; Ganeshkumar, M.; Iyappan, K.; Suguna, L. Efficacy of L-proline administration on the early responses during cutaneous wound healing in rats. Amino Acids 2013, 45, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Voinchet, V.; Giraud-Robert, A.M. Utilisation de l’huile essentielle d’hélichryse italienne et de l’huile végétale de rose musquée après intervention de chirurgie plastique réparatrice et esthétique. Phytothérapie 2007, 5, 67–72. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharm. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Banthia, A.K. Preparation of adrenochrome hydrogel patch, gel, ointment, and the comparison of their blood coagulating and wound healing capability. Mater. Manuf. Process 2006, 21, 297–301. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured: Carol Stream, IL, USA, 2005. [Google Scholar]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef] [Green Version]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Orčić, D.; Anačkov, G.; Balog, K.; Francišković, M.; Mimica-Dukić, N. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti- inflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Valizadeh, A.; Shirzad, M.; Pourmand, M.R.; Farahmandfar, M.; Sereshti, H.; Amani, A. Preparation and comparison of effects of different herbal oil ointments as wound-healing agents. Cells Tissues Organs 2019, 207, 177–186. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, C.; Li, G.; Ding, W.; Zhou, W.; Zhu, H.; Chen, G.; Luo, T.; Guang, M.; Liu, Y.; et al. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp. Anim. 2003, 52, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Petersen, D. Immortelle essential oil and extract: Are two preparations better than one? J. Am. Herb. Guild 2015, 13, 21–27. [Google Scholar]
- Esmaie, E.M.; Abo-Youssef, A.M.; Tohamy, M.A. Antidiabetic and antioxidant effects of tannic acid and melatonin on streptozotocin induced diabetes in rats. Pak. J. Pharm. Sci. 2019, 32, 1453–1459. [Google Scholar] [PubMed]
- Sarandy, M.M.; Novaes, R.D.; Xavier, A.A.; Vital, C.E.; Leite, J.P.V.; Melo, F.C.S.A.; Gonçalves, R.V. Hydroethanolic extract of Strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. BioMed Res. Int. 2017, 2017, 9538351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangavel, P.; Pathak, P.; Kuttalam, I.; Lonchin, S. Effect of ethanolic extract of Melia dubia leaves on full-thickness cutaneous wounds in Wistar rats. Dermatol. Ther. 2019, 32, e13077. [Google Scholar] [CrossRef] [PubMed]
- Andritoiu, C.V.; Andriescu, C.E.; Ibanescu, C.; Lungu, C.; Ivanescu, B.; Vlase, L.; Havarneanu, C.; Popa, M. Effects and characterization of some topical ointments based on vegetal extracts on incision, excision, and thermal wound models. Molecules 2020, 25, 5356. [Google Scholar] [CrossRef]
- Güzel, S.; Özay, Y.; Kumaş, M.; Uzun, C.; Özkorkmaz, E.G.; Yıldırım, Z.; Kahraman, A. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech. f. and Salvia euphratica Montbret, Aucher & Rech. f. var. euphratica on excision and incision wound models in diabetic rats. Biomed. Pharmacother. 2019, 111, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Zaki, M.Z.; Leng, T.M.; Rahman, N.H.; Arshad, S.A.; Hamid, A. Alocasia denudata Engler treatment enhance open wound healing activities in Wistar rat’s skin. J. Ethnopharmacol. 2015, 176, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic derived diallyl trisulfide in experimental metabolic syndrome: Metabolic effects and cardioprotective role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef] [PubMed]







| Peack No. | Compound | RI | % |
|---|---|---|---|
| Monoterpene Hydrocarbons | 18.52 | ||
| 1 | α-Pinene | 937 | 12.38 |
| 2 | Camphene | 952 | 0.43 |
| 3 | β-Pinene | 978 | 0.44 |
| 4 | β-Myrcene | 991 | 0.03 |
| 5 | δ-2-Carene | 1001 | 0.13 |
| 6 | α-Phellandrene | 1005 | 0.06 |
| 7 | δ 3-carene | 1011 | 0.05 |
| 8 | α-Terpinene | 1017 | 0.25 |
| 10 | Limonene | 1030 | 3.74 |
| 12 | cis-β-Ocimene | 1037 | 0.43 |
| 13 | γ-Terpinene | 1060 | 0.45 |
| 14 | Terpinolene | 1088 | 0.13 |
| Aromatic Monoterpene Hydrocarbons | 0.37 | ||
| 9 | p-Cymene | 1025 | 0.37 |
| Oxigenated Monoterpenes | 15.56 | ||
| 11 | 1,8-Cineole | 1032 | 0.23 |
| 16 | Linalool | 1099 | 0.76 |
| 17 | Fenchol | 1113 | 0.13 |
| 19 | endo-Borneol | 1167 | 0.06 |
| 20 | Terpinen-4-ol | 1177 | 0.23 |
| 21 | α-Terpineol | 1189 | 0.28 |
| 22 | Nerol | 1228 | 0.74 |
| 23 | Geraniol | 1253 | 0.17 |
| 23 | Neryl acetate | 1364 | 12.96 |
| Sesquiterpene Hydrocarbons | 59.62 | ||
| 24 | α-Cubebene | 1351 | 2.37 |
| 25 | Ylangene | 1372 | 0.17 |
| 26 | α-Copaene | 1376 | 0.34 |
| 27 | β-Cubenene | 1388 | 0.97 |
| 28 | Italicene | 1403 | 3.58 |
| 29 | cis-α-Bergamotene | 1415 | 0.93 |
| 30 | trans-β-Caryophyllene | 1419 | 4.89 |
| 31 | Cedrene | 1422 | 0.45 |
| 32 | trans-α-Bergamotene | 1435 | 0.88 |
| 33 | Aromandendrene | 1441 | 1.06 |
| 34 | Humulene | 1454 | 0.48 |
| 35 | Alloaromadendrene | 1461 | 2.66 |
| 36 | Acoradiene | 1471 | 0.44 |
| 28 | ar-Curcumene | 1480 | 1.07 |
| 29 | γ-Curcumene | 1483 | 14.07 |
| 40 | β-Selinene | 1486 | 11.27 |
| 41 | α-Selinene | 1494 | 7.27 |
| 42 | δ-Selinene | 1497 | 3.36 |
| 43 | α-Muurolene | 1499 | 0.95 |
| 44 | δ-Cadinene | 1524 | 2.41 |
| Oxigenated Sesquiterpenes | 0.77 | ||
| 45 | Caryophyllene oxide | 1581 | 0.77 |
| 46 | trans-Farnesol | 1725 | 0.26 |
| Aliphatic compounds | 0.34 | ||
| 21 | Dodecane | 1201 | 0.34 |
| Other | 3.19 | ||
| 15 | Butyl angelate | 1091 | 0.29 |
| 18 | 2-Methylbutyl angelate | 1146 | 1.25 |
| 37 | Geranyl propionate | 1475 | 1.65 |
| Total of identified compounds | 98.37 | ||
| Samples | Assay | ||||
|---|---|---|---|---|---|
| DPPH IC50 | OH IC50 | NO IC50 | LP IC50 | FRAP | |
| (µg/mL) | (mg AAE/mL HIEO) | ||||
| HIEO | 4.45 ± 0.44 | 13.33 ± 1.11 | n.d. | 10.48 ± 1.22 | 0.03 ± 0.00 |
| AA | / | 2.03 ± 0.39 | / | / | / |
| PG | 0.69 ± 0.03 | 9.01 ± 0.48 | / | / | / |
| BHT | / | 0.03 ± 0.01 | / | 7.13 ± 0.54 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andjić, M.; Božin, B.; Draginić, N.; Kočović, A.; Jeremić, J.N.; Tomović, M.; Milojević Šamanović, A.; Kladar, N.; Čapo, I.; Jakovljević, V.; et al. Formulation and Evaluation of Helichrysum italicum Essential Oil-Based Topical Formulations for Wound Healing in Diabetic Rats. Pharmaceuticals 2021, 14, 813. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14080813
Andjić M, Božin B, Draginić N, Kočović A, Jeremić JN, Tomović M, Milojević Šamanović A, Kladar N, Čapo I, Jakovljević V, et al. Formulation and Evaluation of Helichrysum italicum Essential Oil-Based Topical Formulations for Wound Healing in Diabetic Rats. Pharmaceuticals. 2021; 14(8):813. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14080813
Chicago/Turabian StyleAndjić, Marijana, Biljana Božin, Nevena Draginić, Aleksandar Kočović, Jovana N. Jeremić, Marina Tomović, Andjela Milojević Šamanović, Nebojša Kladar, Ivan Čapo, Vladimir Jakovljević, and et al. 2021. "Formulation and Evaluation of Helichrysum italicum Essential Oil-Based Topical Formulations for Wound Healing in Diabetic Rats" Pharmaceuticals 14, no. 8: 813. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14080813