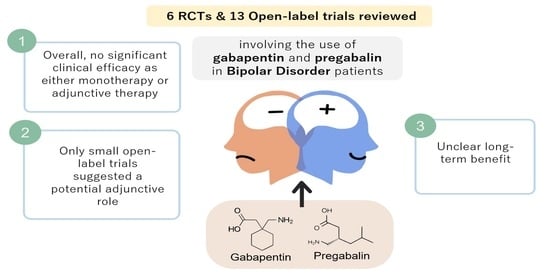
A Systematic Review of the Clinical Use of Gabapentin and Pregabalin in Bipolar Disorder
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Intervention Type
3.2. Dosing Regimes
3.3. Clinical Assessment Tools
3.4. Treatment Efficacy and Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Lamers, F. The ‘true’prevalence of bipolar II disorder. Curr. Opin. Psychiatry 2012, 25, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Benazzi, F. Bipolar disorder—Focus on bipolar II disorder and mixed depression. Lancet 2007, 369, 935–945. [Google Scholar] [CrossRef]
- Schaffer, A.; Isometsä, E.T.; Tondo, L.; Doris, H.M.; Turecki, G.; Reis, C.; Cassidy, F.; Sinyor, M.; Azorin, J.M.; Kessing, L.V. International Society for Bipolar Disorders Task Force on Suicide: Meta-analyses and meta-regression of correlates of suicide attempts and suicide deaths in bipolar disorder. Bipolar Disord. 2015, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, F.; Shen, W.W.; D’ocon, P.; Romero, A.; Álamo, C. A history of the pharmacological treatment of bipolar disorder. Int. J. Mol. Sci. 2018, 19, 2143. [Google Scholar] [CrossRef] [Green Version]
- Serafini, G.; Gonda, X.; Pompili, M.; Rihmer, Z.; Amore, M.; Engel-Yeger, B. The relationship between sensory processing patterns, alexithymia, traumatic childhood experiences, and quality of life among patients with unipolar and bipolar disorders. Child Abus. Negl. 2016, 62, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Budde, K.; Touil, E.; Moock, J.; Bramesfeld, A.; Kawohl, W.; Rössler, W. Cost of illness for bipolar disorder: A systematic review of the economic burden. Bipolar Disord. 2014, 16, 337–353. [Google Scholar] [CrossRef]
- Cloutier, M.; Greene, M.; Guerin, A.; Touya, M.; Wu, E. The economic burden of bipolar I disorder in the United States in 2015. J. Affect. Disord. 2018, 226, 45–51. [Google Scholar] [CrossRef]
- Perlis, R.H.; Ostacher, M.J.; Patel, J.K.; Marangell, L.B.; Zhang, H.; Wisniewski, S.R.; Ketter, T.A.; Miklowitz, D.J.; Otto, M.W.; Gyulai, L. Predictors of recurrence in bipolar disorder: Primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am. J. Psychiatry 2006, 163, 217–224. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Amerio, A.; Russo, D.; Miletto, N.; Aguglia, A.; Costanza, A.; Benatti, B.; Odone, A.; Barroilhet, S.A.; Brakoulias, V.; Dell’Osso, B. Polypharmacy as maintenance treatment in bipolar illness: A systematic review. Acta Psychiatr. Scand. 2021. [Google Scholar] [CrossRef]
- Yamawaki, S.; Kagaya, A.; Tawara, Y.; Inagaki, M. Intracellular calcium signaling systems in the pathophysiology of affective disorders. Life Sci. 1998, 62, 1665–1670. [Google Scholar] [CrossRef]
- Gee, N.S.; Brown, J.P.; Dissanayake, V.U.; Offord, J.; Thurlow, R.; Woodruff, G.N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the subunit of a calcium channel. J. Biol. Chem. 1996, 271, 5768–5776. [Google Scholar] [CrossRef] [Green Version]
- Dooley, D.J.; Donovan, C.M.; Meder, W.P.; Whetzel, S.Z. Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: Inhibition of K+-evoked [3H]-norepinephrine release from rat neocortical slices. Synapse 2002, 45, 171–190. [Google Scholar] [CrossRef]
- Sills, G.J. The mechanisms of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 2006, 6, 108–113. [Google Scholar] [CrossRef]
- Houghton, K.T.; Forrest, A.; Awad, A.; Atkinson, L.Z.; Stockton, S.; Harrison, P.J.; Geddes, J.R.; Cipriani, A. Biological rationale and potential clinical use of gabapentin and pregabalin in bipolar disorder, insomnia and anxiety: Protocol for a systematic review and meta-analysis. BMJ Open 2017, 7, e013433. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altshuler, L.L.; Keck, P.E., Jr.; McElroy, S.L.; Suppes, T.; Brown, E.S.; Denicoff, K.; Frye, M.; Gitlin, M.; Hwang, S.; Goodman, R.; et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord. 1999, 1, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, A.N.; Rezaei, O. Adjunctive treatment with gabapentin in bipolar patients during acute mania. Int. J. Psychiatry Med. 2012, 43, 261–271. [Google Scholar] [CrossRef]
- Cabras, P.L.; Hardoy, J.; Hardoy, M.C.; Carta, M.G. Clinical experience with gabapentin in patients with bipolar or schizoaffective disorder: Results of an open-label study. J. Clin. Psychiatry 1999, 60, 245–248. [Google Scholar] [CrossRef]
- Carta, M.G.; Hardoy, M.C.; Dessi, I.; Hardoy, M.J.; Carpiniello, B. Adjunctive gabapentin in patients with intellectual disability and bipolar spectrum disorders. J. Intellect. Disabil. Res. 2001, 45, 139–145. [Google Scholar] [CrossRef]
- Erfurth, A.; Kammerer, C.; Grunze, H.; Normann, C.; Walden, J. An open label study of gabapentin in the treatment of acute mania. J. Psychiatr. Res. 1998, 32, 261–264. [Google Scholar] [CrossRef]
- Frye, M.A.; Ketter, T.A.; Kimbrell, T.A.; Dunn, R.T.; Speer, A.M.; Osuch, E.A.; Luckenbaugh, D.A.; Cora-Ocatelli, G.; Leverich, G.S.; Post, R.M. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J. Clin. Psychopharmacol. 2000, 20, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Laini, V.; Scalvini, M.E.; Omboni, A.; Ferrari, V.M.; Clemente, A.; Salvi, V.; Cerveri, G. Gabapentin and the prophylaxis of bipolar disorders in patients intolerant to lithium. Clin. Drug Investig. 2001, 21, 169–174. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.L.; Soutullo, C.A.; Keck, P.E., Jr.; Kmetz, G.F. A pilot trial of adjunctive gabapentin in the treatment of bipolar disorder. Ann. Clin. Psychiatry 1997, 9, 99–103. [Google Scholar] [CrossRef]
- Mokhber, N.; Lane, C.J.; Azarpazhooh, M.R.; Salari, E.; Fayazi, R.; Shakeri, M.T.; Young, A.H. Anticonvulsant treatments of dysphoric mania: A trial of gabapentin, lamotrigine and carbamazepine in Iran. Neuropsychiatr. Dis. Treat. 2008, 4, 227–234. [Google Scholar] [PubMed] [Green Version]
- Obrocea, G.V.; Dunn, R.M.; Frye, M.A.; Ketter, T.A.; Luckenbaugh, D.A.; Leverich, G.S.; Speer, A.M.; Osuch, E.A.; Jajodia, K.; Post, R.M. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biol. Psychiatry 2002, 51, 253–260. [Google Scholar] [CrossRef]
- Pande, A.C.; Crockatt, J.G.; Janney, C.A.; Werth, J.L.; Tsaroucha, G. Gabapentin in bipolar disorder: A placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord. 2000, 2, 249–255. [Google Scholar] [CrossRef]
- Perugi, G.; Toni, C.; Frare, F.; Ruffolo, G.; Moretti, L.; Torti, C.; Akiskal, H.S. Effectiveness of adjunctive gabapentin in resistant bipolar disorder: Is it due to anxious-alcohol abuse comorbidity? J. Clin. Psychopharmacol. 2002, 22, 584–591. [Google Scholar] [CrossRef]
- Perugi, G.; Toni, C.; Ruffolo, G.; Sartini, S.; Simonini, E.; Akiskal, H. Clinical experience using adjunctive gabapentin in treatment-resistant bipolar mixed states. Pharmacopsychiatry 1999, 32, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, L.C.; Schaffer, C.B.; Miller, A.R.; Manley, J.L.; Piekut, J.A.; Nordahl, T.E. An open trial of pregabalin as an acute and maintenance adjunctive treatment for outpatients with treatment resistant bipolar disorder. J. Affect. Disord. 2013, 147, 407–410. [Google Scholar] [CrossRef]
- Sokolski, K.N.; Green, C.; Maris, D.E.; DeMet, E.M. Gabapentin as an adjunct to standard mood stabilizers in outpatients with mixed bipolar symptomatology. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 1999, 11, 217–222. [Google Scholar] [CrossRef]
- Vieta, E.; Martinez-Aran, A.; Nieto, E.; Colom, F.; Reinares, M.; Benabarre, A.; Gasto, C. Adjunctive gabapentin treatment of bipolar disorder. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2000, 15, 433–437. [Google Scholar] [CrossRef]
- Vieta, E.; Manuel Goikolea, J.; Martínez-Arán, A.; Comes, M.; Verger, K.; Masramon, X.; Sanchez-Moreno, J.; Colom, F. A double-blind, randomized, placebo-controlled, prophylaxis study of adjunctive gabapentin for bipolar disorder. J. Clin. Psychiatry 2006, 67, 473–477. [Google Scholar] [CrossRef]
- Wang, P.W.; Santosa, C.; Schumacher, M.; Winsberg, M.E.; Strong, C.; Ketter, T.A. Gabapentin augmentation therapy in bipolar depression. Bipolar Disord. 2002, 4, 296–301. [Google Scholar] [CrossRef]
- Young, L.T.; Robb, J.C.; Hasey, G.M.; MacQueen, G.M.; Siotis, I.P.; Marriott, M.; Joffe, R.T. Gabapentin as an adjunctive treatment in bipolar disorder. J. Affect. Disord. 1999, 55, 73–77. [Google Scholar] [CrossRef]
- Post, R.M.; Luckenbaugh, D.A. Unique design issues in clinical trials of patients with bipolar affective disorder. J. Psychiatr. Res. 2003, 37, 61–73. [Google Scholar] [CrossRef]
- Driot, D.; Jouanjus, E.; Oustric, S.; Dupouy, J.; Lapeyre-Mestre, M. Patterns of gabapentin and pregabalin use and misuse: Results of a population-based cohort study in France. Br. J. Clin. Pharmacol. 2019, 85, 1260–1269. [Google Scholar] [CrossRef]
- Leith, W.M.; Lambert, W.E.; Boehnlein, J.K.; Freeman, M.D. The association between gabapentin and suicidality in bipolar patients. Int. Clin. Psychopharmacol. 2019, 34, 27–32. [Google Scholar] [CrossRef]
- Mersfelder, T.L.; Nichols, W.H. Gabapentin: Abuse, dependence, and withdrawal. Ann. Pharmacother. 2016, 50, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, D.Y.; Chee, K.T. Reimagining the spectrum of affective disorders. Bipolar Disord. 2020, 22, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Picardi, A. Rating scales in bipolar disorder. Curr. Opin. Psychiatry 2009, 22, 42–49. [Google Scholar] [CrossRef]
- Miller, C.J.; Johnson, S.L.; Eisner, L. Assessment tools for adult bipolar disorder. Clin. Psychol. Sci. Pract. 2009, 16, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spearing, M.K.; Post, R.M.; Leverich, G.S.; Brandt, D.; Nolen, W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997, 73, 159–171. [Google Scholar] [CrossRef]
- Scott, J.; Murray, G.; Henry, C.; Morken, G.; Scott, E.; Angst, J.; Merikangas, K.R.; Hickie, I.B. Activation in bipolar disorders: A systematic review. JAMA Psychiatry 2017, 74, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Murray, G. Are rating scales for bipolar disorders fit for purpose? Br. J. Psychiatry 2018, 213, 627–629. [Google Scholar] [CrossRef]

| Author, Year | Study Design (N) | Study Population | Clinical Assessment or Rating Tool(s) | Intervention(s) | Key Findings |
|---|---|---|---|---|---|
| Altshuleret al., 1999 [16] | Open-label clinical trial (n = 28 bipolar patients) | Patients had manic (n = 18), depressive (n = 5) or rapid-cycling (n = 5) symptoms that were unresponsive to at least one mood stabiliser. Rapid-cycling patients had treatment initiated when they were euthymic. Patients had consultations with their treating physicians weekly to monthly. | CGI-BP rated every 2 weeks | Gabapentin was given as an adjunctive treatment, added to an existing medicine regimen. Doses ranged from 300 to 3600 mg/day for manic symptoms, 300 to 2400 mg/day for depressive symptoms and 600 to 3000 mg/day for rapid-cycling patients. | - 78% (n = 18) of patients treated for manic symptoms had a positive response to a dose range of 600 to 3600 mg/day - Mean times to a recorded positive response was 12.7 ± 7.2 days for hypomania patients, 25 ± 12 days for classic mania and 31.8 ± 20.9 days for mixed mania. - All 5 patients treated for depression had a positive response in 21 ± 13.9 days, while only 1 patient in the rapid-cycling group had a positive response. - 46% (n = 12) of patients had reported side effects of sedation (n = 5), ataxia (n = 2), dizziness (n = 1) and headache (n = 1) |
| Astaneh et al., 2012 [17] | Randomized, open-label clinical trial (n = 60 bipolar patients) | Patients had a diagnosis of bipolar disorder and were admitted in the acute mania phase. | YMRS rated at the start and the end of therapy | Both groups were treated with lithium for a period of 6 weeks. In the experimental group, gabapentin was given adjunctively (900 mg dose). | - There was significant improvement in the YMRS score of the experimental group as compared to the control group. |
| Cabras et al., 1999 [18] | Open-label clinical trial (n = 25 patients) | Patients were 18 years and older, and had diagnoses of bipolar I disorder (n = 16) or schizoaffective disorder (n = 9) according to the DSM-IV. Patients also had to fulfill DSM-IV criteria for episodes of mania or hypomania. | CGI-S and BPRS evaluated at baseline and every 2 weeks. | Treatment with gabapentin was given over 16 weeks, with other mood stabilisers tapered off over a period of 4 weeks. Baseline prescriptions of benzodiazepines and neuroleptics were maintained. Gabapentin was administered as an initial dose of 300 mg every night, increased by 300 mg/day every 4 days, titrated to patient response and tolerability (maximal dose of 2400 mg/day). | - 76% of patients (n = 19) had a positive response measured by the CGI and BPRS scores. - CGI severity score decreased from 4.0 ± 1.2 at baseline to 2.3 ± 1.1 at week 16. The CGI change score was statistically significant (t = 8.5, df = 21, p < 0.0001). - BPRS score decreased from 29.1 ± 7.1 at baseline to 21.3 ± 3.3 at week 16. The BRPS change score was statistically significant (t = 28.2, df = 11, p < 0.0001). - The mean dose was 1440 mg/day with over-sedation being the most common side effect, as reported in 44% of patients (n = 11). |
| Carta et al., 2001 [19] | Open-label clinical trial (n = 10 patients) | Patients had intellectual disability (ID) with four mild cases, five moderate and one severe. All ten patients had concomitant bipolar disorder (n = 6) or schizo-affective disorder (n = 4). | Assessment and Information Rating Profile (AIRP) with the psychopathology section derived from the Psychopathology Instrument for Mentally Retarded Adults (PIMRA) | Clinical observations were performed during two separate one-month periods, E0 and E1. Following E0, gabapentin was administered adjunctively with doses ranging from 600 to 900 mg/day. Mean treament duration was four months. | - In five patients with affective disorders, there was a statistically significant decrease in total scores at E1 from E0, with improvements recorded in each scale of psychopathology. - Overall scores were 18.6 ± 1.3 during E0 and 10.2 ± 5.8 during E1 (W = 15, p = 0.05, Wilcoxon’s t-test). - The statistical significance was only noted for subscales of anxiety, depression and adjustment disorders. |
| Erfurth et al., 1998 [20] | Open-label trial (n = 14 patients) | Patients met the diagnostic criteria for mania according to the ICD-10. | Bech-Rafaelsen Mania Rating Scale (BMRS) assessed on days 0, 3, 7, 14, and 21 by 2 trained psychiatrists not blind to the treatment | Gabapentin was given as monotherapy for 8 patients and adjunctively for 6 patients for up to 21 days. In the adjunctive group, the existing medication had been administered for a minimum of 14 days without a significant improvement in manic symptoms. Gabapentin dose ranging from 1200 to 4800 mg/day. | - Mean BMRS scores decreased significantly from 37.7 to 7.8 in the adjunctive group, and from 27.8 to 9.0 in four patients who completed monotherapy. - Mild sedation was reported as a side-effect in the adjunctive group (n = 2) who also had concurrent increases in pre-existing medication; may not be due to gabapentin alone. |
| Frye et al., 2000 [21] | Double-blind, randomised, crossover trial (n = 31 patients) | Patients comprised 18 women and 13 men, with bipolar I (n = 11) and bipolar II (n = 14) disorder. 23 had rapid-cycling symptoms while 6 were unipolar patients. | CGI-BP, HAM-D, STAI, YMRS and BPRS | Gabapentin was given as an initial dose of 900 mg/day and increased to 1500 mg/day, 2700 mg/day, 3600 mg/day, 4200 mg/day and 4800 mg/day by the end of the first, second, third, fourth and fifth to sixth weeks, respectively. Patients received 3 treatments (gabapentin, lamotrigine and placebo) sequentially over three 6-week phases with an approximate crossover period of one week between phases. | - 26% of patients (8/31) had positive response rates as denoted by the overall CGI rating after gabapentin administration. - The gabapentin response rates for mania and depression were 20% (5/25) and 26% (8/31), respectively. - Common side effects post-gabapentin administration were ataxia (n = 3), diarrhoea (n = 2), diplopia (n = 3), fatigue (n = 3) and headache (n = 4). |
| Mauri et al., 2001 [22] | Open-label trial (n = 21 patients) | There were 21 outpatients comprising 13 females and 8 males. Patients were diagnosed with bipolar types I and II (according to DSM-IV) and were assessed to be in partial remission. Patients were all intolerant and noncompliant with lithium. | BPRS, HAM-D, HAM-A and Manic Rating Scale (MRS) were assessed at baseline, days 15, 30 and then monthly up to 12 months | Gabapentin was administered at doses ranging from 300 to 2400 mg/day for a period of 1 year. 2 weeks prior to the start of the interventions, all anticonvulsants were ceased, with benzodiazepines used only if necessary. | - Over the one year study period, no significant differences in HAM-D, HAM-A and MRS scores were found. - There was a significant decrease recorded for the mean BPRS scores. - A negative correlation was determined between the dose of gabapentin administered and HAM-A scores (r = 0.16, p = 0.035) but no relationship was found with the mean scores of BPRS, HAM-D and MRS. - No relationship between adverse events and gabapentin dose (mg/kg) observed. |
| McElroy et al., 1997 [23] | Open-label, prospective trial (n = 9 patients) | Patients were 18 years and older; diagnosed with either bipolar I (n = 7) or II (n = 2) according to DSM-IV; did not show adequate response to lithium, valproate or carbamazepine; had symptoms of hypomania, mania or mixed states | Treatment response was evaluated monthly according to this scale: 0 (no response or worsening), 1 (minimal improvement), 2 (moderate improvement) and 3 (marked improvement). | Gabapentin was administered adjunctively as an initial dose of 300 to 900 mg/day, and increased by 300 to 900 mg/day every three to 14 days (titrated to side effects). The maximum dose was 4800 mg/day. | - Seven out of nine patients displayed moderate or marked improvements in symptoms of mania after 1 month of adjunctive gabapentin treatment. - This increased to eight patients after three months of treatment. - Common side effects were sedation (n = 7), forgetfulness (n = 3) and ataxia (n = 2). |
| Mokhber et al., 2008 [24] | Double-blind, fixed dose, randomised clinical trial (n = 59) | Patients comprised 28 women and 3 men; age range between 18 to 60 years; diagnosed with dysphoric mania by DSM-IV; had history of bipolar I disorder with at least one episode of mania prior and a recent episode of mixed mania | Minnesota Multiphasic Personality Inventory 2 (MMPI-2) evaluated at baseline and final visit | Randomisation was performed yielding 3 experimental groups - Group 1 (n = 18): gabapentin 900 mg/day - Group 2 (n = 20): lamotrigine 100 mg/day - Group 3 (n = 13): carbamazepine 600 mg/day | - There was a significant decrease of 50% (p < 0.000) in MMPI-2 scores for depression for the group administered with gabapentin. This decrease was higher than the lamotrigine group (33% decrease) and carbamazepine group (13% decrease). - Similarly, a significant decrease of 75% was recorded for the MMPI-2 scores for mania in the gabapentin group. This decrease was higher than the lamotrigine and cabamazepine groups which had reductions of 64% and 59%, respectively. |
| Obrocea et al., 2002 [25] | Double-blind, three-way, randomized trial (n = 45) | 35 patients with refractory bipolar affective disorder and 10 patients with refractory unipolar affective disorder were recruited in the clinical study, of which there were 27 women and 18 men. | CGI-BP, HAM-D, clinician and self prospective life chart methodology (LCM), YMRS, STAI and Bunney-Hamburg ratings of depression and mania | Gabapentin was administered at a maximum dose of 4800 mg for 6 weeks with a 1 week interval between two subsequent crossovers to the other agents. Lamotrigine (maximum dose of 500 mg) and placebo (equal number of pills to the other drugs) were also administered for the same duration as that of gabapentin. | - Response rates according to overall CGI-BP were 20/39 (51%), 11/40 (28%), and 8/38 (21%) for patients who were administered lamotrigine, gabapentin, and placebo, respectively. - Younger patients responded better than older patients when gabapentin was administered (r = −0.37; p = 0.19). - Patients who had a longer duration of illness responded more poorly than patients who had a shorter duration of illness (r = −0.35; p = 0.19). - Patients who were lighter in weight before the trial responded better to gabapentin than those who were initially heavier (r = −0.44; p = 0.006). - Patients over the age of 45 years and over 95 kg in weight responded poorly to gabapentin, and some patients displayed worsening symptoms. |
| Pande et al., 2000 [26] | Double-blind, placebo-controlled trial(n = 117) | Study cohort comprised outpatients who were diagnosed with bipolar 1 disorder based on DSM-IV criteria, with manic/hypomanic or mixed symptoms. All included patients had to meet the criteria for a lifetime diagnosis of bipolar I and score of more than or equal to 12 on the YMRS at the first clinic visit. | YMRS, HAM-D, HAM-A, CGI-C, Internal state scale (ISS), and Life Chart for Recurrent Affective Illness | 58 patients were administered gabapentin three times a day of a dosage ranging from 600 to 3600 mg/day for 10 weeks, while 59 patients were administered a placebo for the same duration. | - Both treatment groups (gabapentin and placebo) displayed decreased total YMRS scores from baseline to endpoint but this decrease was significantly lower in the gabapentin group (−6) than the placebo group (−9) (p < 0.05). - No difference between treatments were observed for the total score on HAM-D - Secondary efficacy measures were similar between the two treatment groups. |
| Perugi et al., 1999 [27] | Open-label trial (n = 21) | Study cohort comprised patients diagnosed with bipolar type I mixed episodes, based on DSM-III-R criteria. Included patients were resistant to therapeutic levels of standard mood stabilizers and the semistructured interview for mood disorder (SIMD) was utilized to ensure that the diagnostic criteria were satisfied. | HAM-D, YMRS and CGI-C | Gabapentin was administered adjunctively starting with an initial dosage of 300 mg/day which was subsequently increased to 2000 mg/day based on the clinical response and occurrence of any significant side effects. The mean (+/− SD) dose of gabapentin at week 8 was 1130 +/− 361.4 mg (range 600 to 2000 mg). | - Out of the 20 patients who completed the 8 weeks of therapy, 10 were regarded as responders: 4 with a CGI score of 1 (marked improvement) and 6 with a CGI score of 2 (moderate improvement); 9 patients were regarded as nonresponders: 7 with a CGI score of 3 (minimum improvement) and 2 with a CGI score of 4 (no change). - 9 of the 10 responders maintained symptomatic remission over a 4 to 12 month period, without adverse effects. - Mean final CGI score for all patients (responders and nonresponders) was 3.7 + 1.1. - Mean HRSD score showed a statistically significant decrease from 18.2 to 10.6 (t = 5.73; p < 0.0001). |
| Perugi et al., 2002 [28] | Open-label trial (n = 43) | Study cohort comprised patients diagnosed with bipolar disorder and current major depression (n = 14), mixed state (n = 24), or a manic episode (n = 5), based on the DSM-III-R criteria. The SIMD was utilized to ensure that the diagnostic criteria were met. | HAM-D, YMRS and CGI-C | Gabapentin was given as an add-on therapy with other mood stabilizers for 8 weeks. The initial dosage of gabapentin administered was 300 mg/day which was subsequently increased to 2400 mg/day based on the clinical response and occurrence of any significant side effects. The mean (+/− SD) dose of gabapentin at week 8 was 1272 +/− 465.13 mg (range 600 to 2400 mg). | - 18 patients out of the study cohort were considered responders; 8 had a CGI score of 1 and 10 had a CGI score of 2. - 22 patients out of the study cohort were considered non-responders; 15 had a CGI score of 3, 5 had a CGI score of 4, while 5 had a CGI score of 5. - Mean total HAM-D score showed statistically significant reduction during the 8 weeks of treatment from 16.0 to 8.4 (t = 4.51, p > 0.05). - Mean total YMRS score did not show a statistically significant reduction (t = 1.60, p > 0.05). - 17 out of the 18 patients deemed as responders maintained symptomatic remission over a period of 4 to 18 months, without side effects. |
| Schaffer et al., 2013 [29] | Open-label study (n = 58) | All patients (46 females and 12 males; mean age 47 years) were outpatients at a private practice and satisfied the DSM-IV diagnostic criteria for bipolar disorder.Patients were administered pregabalin as an add-on therapy if they were deemed to be nonresponders or unsatisfactory partial responders to majority of the standard medications for bipolar disorder. | CGI-BP | The average dose (+/− SD) of pregabalin for acute responders was 72 mg (+/− 69). The average dose (+/− SD) of pregabalin for non-responders was 84 mg (+/−74). The average dose (+/− SD) of pregabalin for patients on maintenance therapy was 90 mg (+/− 67.9). | - 24/58 patients were deemed as acute responders to pregabalin, of which 12 experienced a mood stabilizing effect of either mixed or rapid cycling symptoms; 5 experienced an antimanic effect; 7 experienced an antidepressant effect. - 10 of these 24 patients were still taking pregabalin as an adjunctive therapy for a mean of 45.2 months (range 42–48; SD: 2.35). |
| Sokolski et al., 1999 [30] | Open-label trial (n = 10) | Outpatients (9 males and 1 female, mean age 50.4 years), diagnosed with Bipolar I by SCID. None were psychotic at entry. Previously received therapeutic dosages of mood stabilizers for at least 2 months with partial responses. | HAM-D and Bech Mania Rating Scales (BMRS) | Adjunctive gabapentin 300 mg initially, increased by 600 mg a week until patients reported a full night sleep or could no longer tolerate sedative side effects. Study duration was 10 weeks. | - Adjunctive gabapentin decreased HAM-D and Bech mania rating scores as early as after the first week of study, and the effects were sustained. - Common side effects include somnolence, dizziness and poor coordination, otherwise well-tolerated. |
| Vieta et al., 2000 [31] | Open-label trial (n = 22) | Twenty-two research diagnostic criteria (RDC) bipolar I (n = 15) and II (n = 7) patients (age >18 years); absence of concomitant serious physical illness; adequate contraceptive control; with presence of at least one episode of the illness in the last six months; presence of residual or subsyndromal features (YMRS > 6 or HAM-D > 12, and CGI-BP > 3); presence of at least one relapse during the treatment with mood stabilizers with serum levels within therapeutic range. | Schedule for Affective Disorders and Schizophrenia (SADS), YMRS and HAM-D scores | Add-on gabapentin were increased by 300 mg/day, titrated to clinical response or tolerance, up to a maximum dose of 2400 mg/day. The mean dose of gabapentin was 1310 mg/day, within a range from 600 to 2400 mg/day. The most common dose prescribed was 1200 mg/day. | - Six patients (27.3%) who did not complete the study dropped out for different reasons: four de to lack of efficacy, one because of intolerance (mild rash) and another because of noncompliance. - 8 patients improved as there was a decrease of at least 2 points in the CGI-BP, in the other eight patients who completed the study, a modest improvement was observed in three of them; four did not show any therapeutic effects. - The comparison of mean scores in CGI-BP showed a significant improvement in the depression subscale that decreased from 4.5 ± 1.2 to 2.9 ± 1.5 points (Wilcoxon Z = −3.1074, p < 0.002), taking into account only patients who completed the study. - The improvement in the mania subscale was not significant (3.3 ± 1.1 vs. 2.9 ± 1.0; Wilcoxon Z = −1.5799, p = NS). - Most patients showed some improvement in social functioning and irritability. - There were non-significant differences in the efficacy of gabapentin between bipolar I and II patients, and between rapid cyclers and non-rapid cyclers. |
| Vieta et al., 2006 [32] | Double-blind, placebo-controlled, randomized trial (n = 25) | Patients, n = 13 in the treatment group (mean age 46.2 years) and n = 12 in the placebo group (mean age 47.6 years), diagnosed with bipolar I or II according to DSM-IV criteria and were treated with any standard mood stabilizer in the last year; two bipolar episodes or more during the last year; CGI-BP scores equal or greater than 4; last episode within past 6 months; euthymic; score of 8 or less on the HAM-D and 4 or less on the YMRS. | CGI-BP, HAM-D, HAM-A, PSQI and YMRS | Gabapentin dose was 1200 mg/day and kept that way unless there were emerging symptoms, then it was increased up to 2400 mg/day and if there were adverse events it would be reduced to 900 mg/day. | - 13 subjects (7 from gabapentin group and 6 from placebo group) completed the study. - Reasons for discontinuation in the gabapentin group were due to withdrawal to participate (n = 2), lack of efficacy (n = 2), adverse events (n = 1) and other reasons (n = 1). In the placebo group, 3 no longer wanted to participate, 1 had a lack of efficacy and 1 had adverse events and 1 (other reasons). - The change in CGI-BP between the groups were statistically significant (gabapentin:−2.1, Placebo: −0.6, p = 0.0046). - No significant differences between groups in YMRS, HAM-D, HAM-A and PSQI scores. - PSQI-6 subscale (use of sleeping medication), the score change at month 12 in the gabapentin group was −1.1 and placebo was −0.6 (p = 0.0267). |
| Wang et al., 2002 [33] | Open-label trial (n = 22) | Outpatients (10 females and 12 males, mean age 38.4 years); met DSM-IV Criteria for bipolar I or II disorder by semistructured clinical interview and DSM-IV criteria for major depressive episode with a 28-item HAM-D score >18 at screening | 28-item HAM-D, YMRS and CGI-S | Adjunctive therapy of gabapentin to stable doses of mood stabilizers or atypical antipsychotics, initiated at 300 mg at bedtime and increased by 300 mg every four nights until symptom relief or adverse effects were noted. Final GBP dose was clinically determined. Maximum dose 3600 mg per day in divided doses (range 600 mg to 3300 mg). | - There was a decrease in mean HAM-D ratings from 32.5 (7.7) to 16.5 (12.8) (t = 8.11, df = 21, p = <0.0001). - Mean CGI-S decreased from 4.4 (0.9) to 3.0 (1.7) (t = 5.2, df = 21, p < 0.0001). - YMRS were unchanged. - 12 of 22 patients were responsive to treatment, with mean HAM-D decreasing 78% from 27.9 (6.2) to 6.2 (4.5), p < 0.0001. 8 of 22 patients were remitted. In non-responders, HAM-D decreased 24% from 38.0 (5.4) to 28.9 (6.7), p = 0.005. - Mild to moderately depressed patients (HAM-D less than 35 at baseline) had a response rate of 77%. Severely depressed patients (HAM-D equal to or greater than 35 at baseline) had a response rate of 22%. Mild to moderately depressed patients had their HAM-D decreased by 16.7 (8.6). Severely depressed patients had their HAM-D decreased by 15.0 (10.7). - Responders had longer bipolar disorder illness duration (23.3 (12.2) vs. 12.9 (9.8)). - Final gabapentin dose was higher in non-responders (2085) than in responders (1425). - Gabapentin was generally well tolerated. Mild sedation was the most common adverse effect in 7 patients. |
| Young et al., 1999 [34] | Open-label trial (n = 37) | Outpatients (12 males and 23 females; mean age 42.2 years), diagnosed with bipolar disorder Type I or II, based on the structured clinical interview for DSM-IV; in both depressed and manic phases. All treated previously with and failed to respond to at least two mood stabilizers. | HAM-D and YMRS | Adjunctive gabapentin to current treatment, dosed 2 to 3 times a day, ranging from 300 mg/day to a maximum of 3600 mg/day and a mean daily dose of 1264 mg (SD: 136). | - Those who were depressed at the start of the study showed a significant decrease in depression symptoms (p < 0.001). This improvement was maintained over 6 months in 17 patients. Significant improvement in the global assessment of functioning from baseline to 12 weeks and 24 weeks (55 +/− 1.3 to 67 +/− 2.9 to 67 +/− 3.6). - In maniac patients, there was a reduction in mania symptoms (p < 0.001) and maintained over 6 months. The manic group showed nonsignificant improvement in global assessment of functioning. - There was a significant overall reduction in anxiety and mood clusters (p < 0.001). - The drug was well tolerated. Side effects include: constipation (n = 4), dry mouth (n = 6), trouble sleeping (n = 7), daytime drowsiness (n = 8), anxiety (n = 9), blurred vision (n = 5), sexual difficulties (n = 9). |
| Study (Author, Year) | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Altshuleret al., 1999 [16] | − | − | − | + | + | ? |
| Astaneh et al., 2012 [17] | + | − | − | ? | ? | ? |
| Cabras et al., 1999 [18] | − | − | − | + | ? | ? |
| Carta et al., 2001 [19] | − | − | − | − | ? | ? |
| Erfurth et al., 1998 [20] | − | − | − | ? | − | − |
| Frye et al., 2000 [21] | + | ? | + | + | + | ? |
| Mauri et al., 2001 [22] | − | − | − | + | ? | ? |
| McElroy et al., 1997 [23] | − | − | − | − | ? | − |
| Mokhber et al., 2008 [24] | ? | ? | + | ? | + | ? |
| Obrocea et al., 2002 [25] | ? | ? | ? | + | + | ? |
| Pande et al., 2000 [26] | ? | ? | ? | + | + | − |
| Perugi et al., 1999 [27] | − | − | − | ? | + | ? |
| Perugi et al., 2002 [28] | − | − | − | ? | + | ? |
| Schaffer et al., 2013 [29] | − | − | − | ? | ? | ? |
| Sokolski et al., 1999 [30] | − | − | − | + | ? | + |
| Vieta et al., 2000 [31] | − | − | − | + | + | + |
| Vieta et al., 2006 [32] | + | + | + | + | + | ? |
| Wang et al., 2002 [33] | − | − | − | + | ? | ? |
| Young et al., 1999 [34] | − | − | − | + | ? | ? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Q.X.; Han, M.X.; Teoh, S.E.; Yaow, C.Y.L.; Lim, Y.L.; Chee, K.T. A Systematic Review of the Clinical Use of Gabapentin and Pregabalin in Bipolar Disorder. Pharmaceuticals 2021, 14, 834. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090834
Ng QX, Han MX, Teoh SE, Yaow CYL, Lim YL, Chee KT. A Systematic Review of the Clinical Use of Gabapentin and Pregabalin in Bipolar Disorder. Pharmaceuticals. 2021; 14(9):834. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090834
Chicago/Turabian StyleNg, Qin Xiang, Ming Xuan Han, Seth En Teoh, Clyve Yu Leon Yaow, Yu Liang Lim, and Kuan Tsee Chee. 2021. "A Systematic Review of the Clinical Use of Gabapentin and Pregabalin in Bipolar Disorder" Pharmaceuticals 14, no. 9: 834. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090834