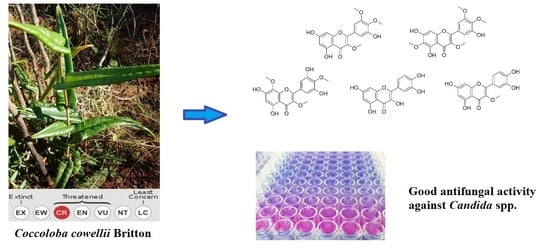
Antifungal Activity of Extracts, Fractions, and Constituents from Coccoloba cowellii Leaves
Abstract
:1. Introduction
2. Results
2.1. Biofractionation Strategy
2.2. UHPLC-HRMS Characterization
2.2.1. Flavonoid Glycosides/Glucuronides
2.2.2. Lignin Oligomers
2.2.3. Methoxyflavonoids
2.3. Isolated Compounds
2.4. Antibiofilm Screening Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals and Plant Material
4.2. Leaf Extraction and Bioassay-Guided Fractionation
4.3. Antifungal Assay
Microorganisms and Dilutions
4.4. Antibiofilm Screening Assay
4.5. Cytotoxicity Assay
4.6. UHPLC-HRMS Characterization
Data Processing
4.7. Isolation of Constituents from Active Fractions
4.8. Structure Elucidation
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Hum. Fungal Pathog. Identif. 2017, 1508, 17–65. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and beyond. Chem. Rev. 2020, 121, 3390–3411. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Méndez, D.; Escalona-Arranz, J.C.; Foubert, K.; Matheeussen, A.; Van der Auwera, A.; Piazza, S.; Cuypers, A.; Cos, P.; Pieters, L. Chemical and Pharmacological Potential of Coccoloba cowellii, an Endemic Endangered Plant from Cuba. Molecules 2021, 26, 935. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2008, 29, 221–235. [Google Scholar] [CrossRef]
- Morreel, K.; Dima, O.; Kim, H.; Lu, F.; Niculaes, C.; Vanholme, R.; Dauwe, R.; Goeminne, G.; Inzé, D.; Messens, E.; et al. Mass Spectrometry-Based Sequencing of Lignin Oligomers. Plant Physiol. 2010, 153, 1464–1478. [Google Scholar] [CrossRef] [Green Version]
- Morreel, K.; Kim, H.; Lu, F.; Dima, O.; Akiyama, T.; Vanholme, R.; Niculaes, C.; Goeminne, G.; Inze, D.; Messens, E.; et al. Mass Spectrometry-Based Fragmentation as an Identification Tool in Lignomics. Anal. Chem. 2010, 82, 8095–8105. [Google Scholar] [CrossRef] [Green Version]
- Kiyota, E.; Mazzafera, P.; Sawaya, A.C.H.F. Analysis of Soluble Lignin in Sugarcane by Ultrahigh Performance Liquid Chromatography—Tandem Mass Spectrometry with a Do-It- Yourself Oligomer Database. Anal. Chem. 2012, 84, 7015–7020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Wang, F.; Zhang, H.; Lu, J.-Q.; Qiao, Y.-J. Rapid Identification of Polymethoxylated Flavonoids in Traditional Chinese Medicines with a Practical Strategy of Stepwise Mass Defect Filtering Coupled to Diagnostic Product Ions Analysis based on a Hybrid LTQ-Orbitrap Mass Spectrometer. Phytochem. Anal. 2014, 25, 405–414. [Google Scholar] [CrossRef]
- El-Kawe, B.M.A. A Pharmacognostical Study of Coccoloba peltata Schott Family Polygonaceae; Cairo University: Cairo, Egypt, 2019. [Google Scholar]
- Nafady, A.; Ibraheim, Z.; Abd El-kader, A.; Ahmed, A. Xanthone and lignan glycosides from the aerial parts of Polygonum bellardii all growing in Egypt. Pharmacogn. Mag. 2013, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Yao, N.; Wang, K.W. Phytochemical and chemotaxomic study on Polygonum perfoliatum L. Biochem. Syst. Ecol. 2013, 48, 186–188. [Google Scholar] [CrossRef]
- Wang, K.W.; Zhu, J.R.; Shen, L.Q. A new lignan with anti-tumour activity from Polygonum perfoliatum L. Nat. Prod. Res. 2013, 27, 568–573. [Google Scholar] [CrossRef]
- Cong, H.J.; Zhang, S.W.; Zhang, C.; Huang, Y.J.; Xuan, L.J. A novel dimeric procyanidin glucoside from Polygonum aviculare. Chin. Chem. Lett. 2012, 23, 820–822. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Münzbergová, Z.; Timsina, B.; Bhattarai, K.R. Rheum australe D. Don: A review of its botany, ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2012, 141, 761–774. [Google Scholar] [CrossRef]
- Odonbayar, B.; Murata, T.; Batkhuu, J.; Yasunaga, K.; Goto, R.; Sasaki, K. Antioxidant Flavonols and Phenolic Compounds from Atraphaxis frutescens and Their Inhibitory Activities against Insect Phenoloxidase and Mushroom Tyrosinase. J. Nat. Prod. 2016, 79, 3065–3071. [Google Scholar] [CrossRef]
- Huang, G.H.; Gao, Y.; Wu, Z.J.; Yang, Y.; Huang, D.D.; Chen, W.S.; Sun, L.N. Chemical constituents from Polygonum capitatum Buch-Ham. ex D. Don. Biochem. Syst. Ecol. 2015, 59, 8–11. [Google Scholar] [CrossRef]
- Chung, H.S.; Chang, L.C.; Lee, S.K.; Shamon, L.A.; Van Breemen, R.B.; Mehta, R.G.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Flavonoid constituents of Chorizanthe diffusa with potential cancer chemopreventive activity. J. Agric. Food Chem. 1999, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Su, Y.; Yan, S.; Wu, Z.; Zhang, X.; Wang, T.; Gao, X. Hexaoxygenated Flavonoids from Pteroxygonum giraldii. Nat. Prod. Commun. 2010, 5, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Rao Ravu, R.; Tekwani, B.L.; Li, W.; Liu, W.B.; Jacob, M.R.; Khan, S.I.; Cai, X.; Peng, C.Y.; Khan, I.A.; et al. Biological evaluation of phytoconstituents from Polygonum hydropiper. Nat. Prod. Res. 2017, 31, 2053–2057. [Google Scholar] [CrossRef]
- Datta, B.K.; Datta, S.K.; Rashid, M.A.; Nash, R.J.; Sarker, S.D. A sesquiterpene acid and flavonoids from Polygonum viscosum. Phytochemistry 2000, 54, 201–205. [Google Scholar] [CrossRef]
- Maury, G.L.; Rodríguez, D.M.; Hendrix, S.; Arranz, J.C.E.; Boix, Y.F.; Pacheco, A.O.; Díaz, J.G.; Morris-Quevedo, H.J.; Dubois, A.F.; Aleman, E.I.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef]
- Lallemand, J.Y.; Duteil, M. 13C n.m.r. spectra of quercetin and rutin. Org. Magn. Reson. 1977, 9, 179–180. [Google Scholar] [CrossRef]
- Schwingel, L.C.; Schwingel, G.O.; Storch, N.; Barreto, F.; Bassani, V.L. 3-O-Methylquercetin from organic Nicotiana tabacum L. trichomes: Influence of the variety, cultivation and extraction parameters. Ind. Crops Prod. 2014, 55, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Rabesa, Z.A.; Voirin, B. ouveaux aglycones flavoniques O-methyles derives de la mearnsetine chez Alluaudia ascendens. Phytochemistry 1979, 18, 360–362. [Google Scholar] [CrossRef]
- Ayanoglu, E.; Ulubelen, A.; Clark, W.D.; Brown, G.K.; Kerr, R.R.; Mabry, T.J. Myricetin and quercetin methyl ethers from Haplopappus integerrimus var. Punctatus. Phytochemistry 1981, 20, 1715–1717. [Google Scholar] [CrossRef]
- Mai, L.H.; Chabot, G.G.; Grellier, P.; Quentin, L.; Dumontet, V.; Poulain, C.; Espindola, L.S.; Michel, S.; Vo, H.T.B.; Deguin, B.; et al. Antivascular and anti-parasite activities of natural and hemisynthetic flavonoids from New Caledonian Gardenia species (Rubiaceae). Eur. J. Med. Chem. 2015, 93, 93–100. [Google Scholar] [CrossRef]
- Fang, N.; Leidig, M.; Mabry, T.J. Fifty-one flavonoids from Gutierrezia microcephala. Phytochemistry 1986, 25, 927–934. [Google Scholar] [CrossRef]
- Rabesa, Z.A.; Voirin, B. Deux nouveaux aglycones flavoniques isolés de Decaryiamada gascariensis. Phytochemistry 1980, 19, 710–711. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Cheng, D.; He, M.; Pan, S.; Yao, X.; Xu, X. Antifungal action and inhibitory mechanism of polymethoxylated flavones from Citrus reticulata Blanco peel against Aspergillus niger. Food Control 2014, 35, 354–359. [Google Scholar] [CrossRef]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Mangoyi, R.; Midiwo, J.; Mukanganyama, S. Isolation and characterization of an antifungal compound 5-hydroxy-7,4′-dimethoxyflavone from Combretum zeyheri. BMC Complement. Altern. Med. 2015, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Robertson, S.N.; Williams, C. Strength in numbers: Antifungal strategies against fungal biofilms. Int. J. Antimicrob. Agents 2014, 43, 114–120. [Google Scholar] [CrossRef]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Nothias, L.F.; Nothias-Esposito, M.; Da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, A.L.D.; Kaplum, V.; Rossi, D.C.P.; da Silva, L.B.R.; Melhem, M. de S.C.; Taborda, C.P.; de Mello, J.C.P.; Nakamura, C.V.; Ishida, K. Proanthocyanidin polymeric tannins from Stryphnodendron adstringens are effective against Candida spp. isolates and for vaginal candidiasis treatment. J. Ethnopharmacol. 2018, 216, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.R.; De Andrade Neto, J.B.; De Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.F.; Cavalcanti, B.C.; Gaspar, D.M.; De Andrade, G.M.; Lima, I.S.P.; et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y. Synergic anticandidal effect of epigallocatechin-O-gallate combined with amphotericin B in a murine model of disseminated candidiasis and its anticandidal mechanism. Biol. Pharm. Bull. 2007, 30, 1693–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- de Cremer, K.; Lanckacker, E.; Cools, T.L.; Bax, M.; de Brucker, K.; Cos, P.; Cammue, B.P.A.; Thevissen, K. Artemisinins, new miconazole potentiators resulting in increased activity against Candida albicans biofilms. Antimicrob. Agents Chemother. 2015, 59, 421–426. [Google Scholar] [CrossRef] [Green Version]



| Test Sample | Cytotoxicity (CC50 µg/mL) | Antifungal Screening (IC50 µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| MRC-5 | Aspergillus fumigatus | Cryptococcus neoformans | Candida albicans | Candida parapsilosis | Candida glabrata | Candida tropicalis | |
| TE | >64.0 | >64.0 | 2.7 ± 2.0 | 1.7 ± 0.6 | 8.5 ± 0.5 | 0.4 ± 0.0 | 21.2 ± 1.8 |
| MeOH90-F | >64.0 | >64.0 | 10.5 ± 1.0 | 8.3 ± 0.9 | 13.3 ± 1.1 | 2.4 ± 0.4 | >64.0 |
| nH-F | 29.3 ± 1.5 | Nd | >64.0 | >64.0 | Nd | Nd | Nd |
| EtOAc-F | >64.0 | Nd | >64.0 | >64.0 | Nd | Nd | Nd |
| nBut-F | >64.0 | Nd | >64.0 | >64.0 | Nd | Nd | Nd |
| Re-F | >64.0 | Nd | >64.0 | >64.0 | Nd | Nd | Nd |
| Miconazole | 19.8 ± 0.7 | 3.7 ± 0.5 | 0.2 ± 0.0 | 3.4 ± 0.2 | 1.1 ± 0.1 | 0.2 ± 0.0 | 3.6 ± 1.0 |
| Peak No. | Rt (min) | [M-H]− (m/z) | Theoretical Mass (m/z) | Accuracy (ppm) | MS/MS Ions | MF | Tentative Identification |
|---|---|---|---|---|---|---|---|
| 1 | 10.39 | 493.0620 | 493.0618 | 0.4 | 317.0281/315.0105/287.0563/178.9872 | C21H17O14 | Myricetin-O-glucuronide |
| 2 | 12.33 | 433.0775 | 433.0771 | 0.9 | 301.0344/300.0273/271.0247/255.0294 | C20H17O11 | Quercetin-O-pentoside 1 |
| 3 | 12.47 | 433.0763 | 433.0771 | −1.8 | 301.0357/300.0253/271.0255/255.0187 | C20H17O11 | Quercetin-O-pentoside 2 |
| 4 | 13.23 | 555.2225 | 555.2230 | −0.9 | 507.2011/477.1888 | C30H35O10 | Trilignol G(8–O–4)G(8–5)G |
| 5 | 13.50 | 555.2216 | 555.2230 | −2.5 | 507.1984/477.1816/341.1288/329.1320/195.0650/165.0273 | C30H35O10 | Trilignol G(8–O–4)G(8–5)G |
| 6 | 13.85 | 312.1228 | 312.1236 | −2.6 | 197.8091/195.8118/116.9287 | - | Unknown |
| 7 | 14.49 | 585.2429 | 585.2430 | −0.2 | 537.2122/507.1984/371.1458/359.1454/195.0658/165.0374 | C31H37O11 | Trilignol G(8–O–4)X(8–8)X |
| 8 | 14.56 | 583.2163 | 583.2179 | −2.7 | 535.1965/505.1852/369.1333/357.1330/195.0658/165.0301 | C31H35O11 | Trilignol G(8–O–4)S(8–5)G |
| 9 | 14.82 | 585.2329 | 585.2336 | −1.2 | 537.2112/507.1821/359.1410/195.0639/165.0157 | C31H37O11 | Trilignol G(8–O–4)X(8–8)X |
| 10 | 15.09 | 585.2331 | 585.2336 | −0.9 | 537.2020/507.1826/371.1437/359.1445/195.0655/165.0552 | C31H37O11 | Trilignol G(8–O–4)X(8–8)X |
| 11 | 15.17 | 583.2172 | 583.2179 | −1.2 | 369.1325/357.1325/195.0656/165.0551 | C31H35O11 | Trilignol G(8–O–4)S(8–5)G |
| 12 | 15.76 | 583.2177 | 583.2179 | −0.3 | 565.2036/489.1883/477.1877/417.1481/371.1414/359.1383/193.0497 | - | Unknown |
| 13 | 16.02 | 583.2177 | 583.2179 | −0.3 | 581.1965/535.1947/387.1389/367.1148/195.0648/165.0052 | - | Unknown |
| 14 | 16.12 | 315.0513 | 315.0505 | 2.5 | 300.0270/271.0238 | C16H11O7 | 3-O-Methylquercetin |
| 15 | 16.24 | 375.0704 | 375.0716 | −3.2 | 360.0495/345.0239/330.0117/327.1691/317.0265/300.0250/171.0929 | C18H15O9 | 6-Methoxymyricetin 3,4′-dimethyl ether |
| 16 | 16.37 | 327.2177 | 327.2171 | 1.8 | 285.0412/256.0378/229.1443/211.1334/171.1033 | - | Unknown |
| 17 | 16.49 | 345.0612 | 345.0610 | 0.6 | 301.0422 | - | Unknown |
| 18 | 16.71 | 315.0510 | 315.0505 | 1.6 | 300.0278/271.0252/255.0304/243.0296 | C16H11O7 | O-Methylquercetin |
| 19 | 16.85 | 809.3019 | 809.3021 | −0.2 | 761.2747/613.2260/565.2047/417.1499/195.0660 | C42H49O16 | Tetralignol G(8–O–4)G(8–O–4)S(8–8)S |
| 20 | 17.11 | 331.2645 | 331.2637 | 2.4 | 313.2187 | - | Unknown |
| 21 | 17.73 | 389.0888 | 389.0873 | 3.9 | 374.0632/359.0416/331.0509/316.0201/287.2135 | C19H17O9 | 6-Methoxymyricetin 3,3′,4′-trimethyl ether |
| 22 | 17.99 | 359.0764 | 359.0767 | −0.8 | 344.0509/329.0413/301.0361/286.0089/273.0367/257.9776/242.0100/222.9688/162.8474 | C18H15O8 | Myricetin 3,3′,4′-trimethyl ether |
| 23 | 18.13 | 359.0751 | 359.0767 | −4.5 | 344.0493/329.0565/301.0364/286.0081/257.9645/222.9675/162.8543 | C18H15O8 | Methoxyquercetin dimethyl ether 1 |
| 24 | 18.25 | 389.0870 | 389.0873 | −0.8 | 374.0634/359.0398/344.0168/316.0218/300.9995/245.0086 | C19H17O9 | Methoxymyricetin trimethyl ether |
| 25 | 18.48 | 359.0771 | 359.0767 | 1.1 | 344.0535/329.0302/301.0346/286.0122/258.0163 | C18H15O8 | Methoxyquercetindimethyl ether 2 |
| 26 | 19.58 | 403.1047 | 403.1029 | 4.5 | 388.0773/373.0557/358.0301/345.0566/330.0363/315.0175/257.9344/222.9669 | C20H19O9 | Methoxymyricetin tetramethyl ether |
| 27 | 19.83 | 373.0939 | 373.0923 | 4.3 | 358.0623/343.0453/328.0199/315.0660/300.0232/285.0035/257.9385/222.9662 | C19H17O8 | Myricetin tetramethyl ether |
| 28 | 20.18 | 349.2156 | 349.2168 | −3.4 | 313.2335/251.1598/199.8060/197.8089/195.8118/116.9286 | - | Unknown |
| 29 | 21.03 | 721.3658 | 721.3647 | 1.5 | 675.3555/415.1435/397.1342/277.1996/257.9326/222.9646 | - | Unknown |
| 30 | 22.15 | 559.3133 | 559.3118 | 2.7 | 567.2234/505.1054/320.0494/277.2092/257.9327/222.9659 | - | Unknown |
| Ions | Peak 4 | Peak 5 | Peak 7 | Peak 8 | Peak 9 | Peak 10 | Peak 11 |
|---|---|---|---|---|---|---|---|
| [M-H]− | 555 (18) | 555 (49) | 585 (41) | 583 (38) | 585 (36) | 585 (58) | 583 (45) |
| [M-H-H2O]− | 537 (4) | 537 (12) | 567 (2) | 565 (3) | 567 (2) | 567 (4) | 565 (7) |
| [M-H-H2O-CH2O]− | 507 (100) | 507 (90) | 537 (100) | 535 (100) | 537 (100) | 537 (18) | 535 (15) |
| [M-H-H2O-CH2O-CH2O]− | 477 (27) | 477 (55) | 507 (23) | 505 (20) | 507 (13) | 507 (12) | 505 (10) |
| A− | 195 (13) | 195 (100) | 195 (27) | 195 (37) | 195 (32) | 195 (100) | 195 (100) |
| A−-CH2O | 165 (13) | 165 (78) | 165 (19) | 165 (31) | 165 (22) | 165 (67) | 165 (70) |
| B−-H2O | 341 (7) | 341 (46) | 371 (24) | 369 (41) | 371 (3) | 371 (66) | 369 (90) |
| B−-CH2O | 329 (10) | 329 (77) | 359 (30) | 357 (39) | 359 (50) | 359 (82) | 357 (83) |
| Ions | Peak 14 | Peak 15 | Peak 18 | Peak 21 | Peak 22 | Peak 23 | Peak 24 | Peak 25 | Peak 26 | Peak 27 |
|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]− | 315 (15) | 375 (12) | 315 (25) | 389 (19) | 359 (39) | 359 (19) | 389 (12) | 359 (12) | 403 (19) | 373 (11) |
| [M-H-CH3.]− | 300 (100) | 360 (36) | 300 (66) | 374 (44) | 344 (65) | 344 (40) | 374 (22) | 344 (7) | 388 (54) | 358 (11) |
| [M-H-2CH3.]− | - | 345 (100) | - | 359 (100) | 329 (100) | 329 (100) | 359 (100) | 329 (100) | 373 (100) | 343 (100) |
| [M-H-3CH3.]− | - | 330 (17) | - | 344 (13) | 314 (15) | 314 (16) | 344 (36) | 314 (7) | 358 (34) | 328 (12) |
| [M-H-2CH3.-CO]− | - | 317 (15) | - | 331 (38) | 301 (54) | 301 (49) | 331 (7) | 301 (16) | 345 (33) | 315 (10) |
| [M-H-2CH3.-CO-CH3.]− | - | - | - | 316 (32) | 286 (42) | 286 (43) | 316 (44) | 286 (19) | 330 (69) | 300 (13) |
| [M-H-2CH3.-CO-2CH3.]− | - | - | - | 301 (17) | - | - | 301 (16) | - | 315 (34) | 285 (7) |
| [M-H-2CH3.-H2O]− | - | 327 (24) | - | 341 (17) | - | - | - | - | - | - |
| [M-H-CH3.-HCO.]− | 271 (12) | - | 271 (100) | - | - | - | - | - | - | - |
| Others | 300 (22) 171 (24) | 255 (42) 243 (20) | 287 (62) 245 (15) | 273 (46) 258 (62) | 258 (48) 223 (22) 163 (18) | 245 (12) | 258 (14) | 258 (37) |
| Test Sample | Cytotoxicity (CC50 µg/mL) | Antifungal Screening (IC50 µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| MRC-5 | A. fumigatus | C. neoformans | C. albicans | C. parapsilosis | C. glabrata | C. tropicalis | |
| M-6 | >64.0 | >64.0 | 50.3 ± 9.2 | >64.0 | >64.0 | 9.5 ± 1.1 | >64.0 |
| M-6A | >64.0 | >64.0 | >64.0 | >64.0 | >64.0 | 7.9 ± 1.3 | >64.0 |
| M-6B | >64.0 | >64.0 | 59.5 ± 6.4 | >64.0 | >64.0 | 9.1 ± 1.8 | >64.0 |
| M-6C | >32.0 | >32.0 | 8.3 ± 0.0 | >32.0 | >32.0 | 3.8 ± 0.0 | >32.0 |
| Miconazole | 19.8 ± 0.7 | 3.7 ± 0.5 | 0.2 ± 0.0 | 3.4 ± 0.2 | 1.1 ± 0.1 | 0.2 ± 0.0 | 3.6 ± 1.0 |
| Test Sample | Antibiofilm Screening (IC50 µg/mL) |
|---|---|
| Candida albicans SC5314 | |
| TE | 49.73 ± 2.1 |
| MeOH90-F | >64.00 |
| M-6 | >64.00 |
| M-6A | >64.00 |
| M-6B | >64.00 |
| M-6C | >32.00 |
| Miconazole | 0.60 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez, D.; Escalona-Arranz, J.C.; Pérez, E.M.; Foubert, K.; Matheeussen, A.; Tuenter, E.; Cuypers, A.; Cos, P.; Pieters, L. Antifungal Activity of Extracts, Fractions, and Constituents from Coccoloba cowellii Leaves. Pharmaceuticals 2021, 14, 917. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090917
Méndez D, Escalona-Arranz JC, Pérez EM, Foubert K, Matheeussen A, Tuenter E, Cuypers A, Cos P, Pieters L. Antifungal Activity of Extracts, Fractions, and Constituents from Coccoloba cowellii Leaves. Pharmaceuticals. 2021; 14(9):917. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090917
Chicago/Turabian StyleMéndez, Daniel, Julio C. Escalona-Arranz, Enrique Molina Pérez, Kenn Foubert, An Matheeussen, Emmy Tuenter, Ann Cuypers, Paul Cos, and Luc Pieters. 2021. "Antifungal Activity of Extracts, Fractions, and Constituents from Coccoloba cowellii Leaves" Pharmaceuticals 14, no. 9: 917. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090917