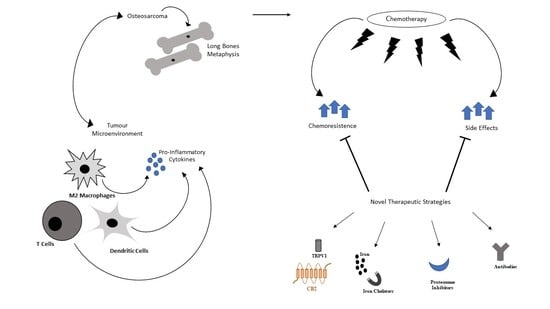
Osteosarcoma in Children: Not Only Chemotherapy
Abstract
:1. Introduction
2. Therapy in OS
3. Proteasome Inhibitors and Their Potential in OS
4. Endocannabinoid/Endovanilloid System in OS
5. Immunotherapy and Its Potential in OS
6. Iron Chelation Effects in OS
7. Emerging Anti-Neoplastic Drugs in OS
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleinerman, E.S.; Mary, V.; John, A. Osteosarcoma: The state of affairs dictates a change. What do we know? Adv. Exp. Med. Biol. 2014, 804, vii–viii. [Google Scholar] [PubMed]
- McGuire, J.; Utset-Ward, T.J.; Reed, D.R.; Lynch, C.C. Re-calculating! Navigating through the osteosarcoma treatment roadblock. Pharmacol. Res. 2017, 117, 54–64. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Zambo, I.; Vesely, K. WHO classification of tumours of soft tissue and bone 2013: The main changes compared to the 3rd edition. Ceskoslovenska Patol. 2014, 50, 64–70. [Google Scholar]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex but Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goryn, T.; Szostakowski, B.; Pienkowski, A.; Zdzienicki, M.; Lugowska, I.; Rutkowski, P. Long-term follow-up in adults with extremity osteosarcoma: Comparison of different surgical procedures—Single-center experience. Contemp. Oncol. 2019, 23, 234–238. [Google Scholar]
- Gill, J.; Ahluwalia, M.K.; Geller, D.; Gorlick, R. New targets and approaches in osteosarcoma. Pharmacol. Ther. 2013, 137, 89–99. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, N.; Puri, A.; Gelderblom, H. Osteosarcoma: Evolution of treatment paradigms. Sarcoma 2013, 2013, 203531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Aljubran, A.H.; Griffin, A.; Pintilie, M.; Blackstein, M. Osteosarcoma in adolescents and adults: Survival analysis with and without lung metastases. Ann. Oncol. 2009, 20, 1136–1141. [Google Scholar] [CrossRef]
- Meyers, P.A.; Gorlick, R.; Heller, G.; Casper, E.; Lane, J.; Huvos, A.G.; Healey, J.H. Intensification of preoperative chemotherapy for osteogenic sarcoma: Results of the Memorial Sloan-Kettering (T12) protocol. J. Clin. Oncol. 1998, 16, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; De Luca, A.; Gallo, A.; Costa, V.; Russelli, G.; Cuscino, N.; Manno, M.; Raccosta, S.; Carina, V.; Bellavia, D.; et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis 2020, 41, 666–677. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Inagaki, Y.; Hookway, E.; Williams, K.A.; Hassan, A.B.; Oppermann, U.; Tanaka, Y.; Soilleux, E.; Athanasou, N.A. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin. Sarcoma Res. 2016, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.; Burger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; Larousserie, F.; Entz-Werle, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology 2017, 6, e1331193. [Google Scholar]
- Jimmy, R.; Stern, C.; Lisy, K.; White, S. Effectiveness of mifamurtide in addition to standard chemotherapy for high-grade osteosarcoma: A systematic review. Syst. Rev. Implement. Rep. 2017, 15, 2113–2152. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, R.; Fourman, M.S.; Bhogal, S.; Weiss, K.R. Update on Osteosarcoma. Curr. Oncol. Rep. 2021, 23, 71. [Google Scholar] [CrossRef]
- Schiavone, K.; Garnier, D.; Heymann, M.F.; Heymann, D. The Heterogeneity of Osteosarcoma: The Role Played by Cancer Stem Cells. Adv. Exp. Med. Biol. 2019, 1139, 187–200. [Google Scholar] [PubMed]
- Zhou, C.; Zhang, Z.; Zhu, X.; Qian, G.; Zhou, Y.; Sun, Y.; Yu, W.; Wang, J.; Lu, H.; Lin, F.; et al. N6-Methyladenosine modification of the TRIM7 positively regulates tumorigenesis and chemoresistance in osteosarcoma through ubiquitination of BRMS1. EBioMedicine 2020, 59, 102955. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, R.; Li, J.; Lu, X.; Zhang, Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: A network meta-analysis. J. Orthop. Surg. Res. 2020, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, D.C.; Carney, S.C.; Ahlmann, E.R.; Hendifar, A.; Chawla, S.; Fedenko, A.; Angeles, C.; Menendez, L.R. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 2012, 704872. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhao, L.; Zhang, Y.; Li, F.F. A comprehensive analysis of immune infiltration in the tumor microenvironment of osteosarcoma. Cancer Med. 2021, 10, 5696–5711. [Google Scholar] [CrossRef]
- Ozkurt, B.; Basarir, K.; Yalcin, B.; Merter, A.; Yildiz, Y.; Saglik, Y. Chemotherapy in primary osteogenic sarcoma in patients over the age of forty. Acta Orthop. Traumatol. Turc. 2017, 51, 123–127. [Google Scholar] [CrossRef]
- Meyers, P.A.; Healey, J.H.; Chou, A.J.; Wexler, L.H.; Merola, P.R.; Morris, C.D.; Laquaglia, M.P.; Kellick, M.G.; Abramson, S.J.; Gorlick, R. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer 2011, 117, 1736–1744. [Google Scholar] [CrossRef] [Green Version]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Liu, P.P.; Wang, Z.X.; Chen, C.Y.; Xie, H. Macrophages in Osteosarcoma Immune Microenvironment: Implications for Immunotherapy. Front. Oncol. 2020, 10, 586580. [Google Scholar] [CrossRef]
- Bellini, G.; Di Pinto, D.; Tortora, C.; Manzo, I.; Punzo, F.; Casale, F.; Rossi, F. The Role of Mifamurtide in Chemotherapy-induced Osteoporosis of Children with Osteosarcoma. Curr. Cancer Drug Targets 2017, 17, 650–656. [Google Scholar] [CrossRef]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef] [Green Version]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Thibaudeau, T.A.; Smith, D.M. A Practical Review of Proteasome Pharmacology. Pharmacol. Rev. 2019, 71, 170–197. [Google Scholar] [CrossRef] [Green Version]
- Varga, C.; Laubach, J.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. Novel targeted agents in the treatment of multiple myeloma. Hematol. Oncol. Clin. N. Am. 2014, 28, 903–925. [Google Scholar] [CrossRef]
- Perkins, S.M.; Dewees, T.; Shinohara, E.T.; Reddy, M.M.; Frangoul, H. Risk of subsequent malignancies in survivors of childhood leukemia. J. Cancer Surviv. 2013, 7, 544–550. [Google Scholar] [CrossRef]
- McConkey, D.J. The integrated stress response and proteotoxicity in cancer therapy. Biochem. Biophys Res. Commun. 2017, 482, 450–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Handa, H.; Chou, T.; Ishizawa, K.; Takubo, T.; Kase, Y. Phase 1 study of ixazomib alone or combined with lenalidomide-dexamethasone in Japanese patients with relapsed/refractory multiple myeloma. Int. J. Hematol. 2017, 105, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fu, C.; Sun, J.; Wang, X.; Geng, S.; Wang, X.; Zou, J.; Bi, Z.; Yang, C. A New Perspective for Osteosarcoma Therapy: Proteasome Inhibition by MLN9708/2238 Successfully Induces Apoptosis and Cell Cycle Arrest and Attenuates the Invasion Ability of Osteosarcoma Cells in Vitro. Cell. Physiol. Biochem. 2017, 41, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Augello, G.; Modica, M.; Azzolina, A.; Puleio, R.; Cassata, G.; Emma, M.R.; Di Sano, C.; Cusimano, A.; Montalto, G.; Cervello, M. Preclinical evaluation of antitumor activity of the proteasome inhibitor MLN2238 (ixazomib) in hepatocellular carcinoma cells. Cell Death Dis. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engur, S.; Dikmen, M. The evaluation of the anti-cancer activity of ixazomib on Caco2 colon solid tumor cells, comparison with bortezomib. Acta Clin. Belg. 2017, 72, 391–398. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Hu, T.; Wang, L.; Yu, Y.; Zhao, Y.; Sun, W.; Guan, S.; Pang, J.C.; Woodfield, S.E.; et al. Novel proteasome inhibitor ixazomib sensitizes neuroblastoma cells to doxorubicin treatment. Sci. Rep. 2016, 6, 34397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Dou, Q.P. The ubiquitin-proteasome system as a prospective molecular target for cancer treatment and prevention. Curr. Protein Pept. Sci. 2010, 11, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Raedler, L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am. Health Drug Benefits 2015, 8, 135–140. [Google Scholar]
- Zavrski, I.; Kleeberg, L.; Kaiser, M.; Fleissner, C.; Heider, U.; Sterz, J.; Jakob, C.; Sezer, O. Proteasome as an emerging therapeutic target in cancer. Curr. Pharm. Des. 2007, 13, 471–485. [Google Scholar] [CrossRef]
- Von Metzler, I.; Krebbel, H.; Hecht, M.; Manz, R.A.; Fleissner, C.; Mieth, M.; Kaiser, M.; Jakob, C.; Sterz, J.; Kleeberg, L.; et al. Bortezomib inhibits human osteoclastogenesis. Leukemia 2007, 21, 2025–2034. [Google Scholar] [CrossRef]
- Giuliani, N.; Morandi, F.; Tagliaferri, S.; Lazzaretti, M.; Bonomini, S.; Crugnola, M.; Mancini, C.; Martella, E.; Ferrari, L.; Tabilio, A.; et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 2007, 110, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, T.; Kawashima, H.; Ogose, A.; Ariizumi, T.; Sasaki, T.; Hatano, H.; Hotta, T.; Endo, N. Receptor-Activator of Nuclear KappaB Ligand Expression as a New Therapeutic Target in Primary Bone Tumors. PLoS ONE 2016, 11, e0154680. [Google Scholar] [CrossRef]
- Sisay, M.; Mengistu, G.; Edessa, D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: Potential targets for anticancer therapy. OncoTargets Ther. 2017, 10, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Barwick, B.G.; Shanmugam, M.; Hofmeister, C.C.; Kaufman, J.; Nooka, A.; Gupta, V.; Dhodapkar, M.; Boise, L.H.; Lonial, S. Downregulation of PA28alpha induces proteasome remodeling and results in resistance to proteasome inhibitors in multiple myeloma. Blood Cancer J. 2020, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Muscal, J.A.; Thompson, P.A.; Horton, T.M.; Ingle, A.M.; Ahern, C.H.; McGovern, R.M.; Reid, J.M.; Ames, M.M.; Espinoza-Delgado, I.; Weigel, B.J.; et al. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: A Children’s Oncology Group phase I consortium study (ADVL0916). Pediatr. Blood Cancer 2013, 60, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 134, 2534–2546. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543–77557. [Google Scholar] [CrossRef] [Green Version]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Pota, E.; Argenziano, M.; Di Paola, A.; Casale, F.; Rossi, F. Bortezomib and endocannabinoid/endovanilloid system: A synergism in osteosarcoma. Pharmacol. Res. 2018, 137, 25–33. [Google Scholar] [CrossRef]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Manzo, I.; Bellini, G.; Casale, F.; Rossi, F. Anti-proliferative, pro-apoptotic and anti-invasive effect of EC/EV system in human osteosarcoma. Oncotarget 2017, 8, 54459–54471. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, J.; Watson, J.E.; Hong, I.S.; Fan, T.M.; Das, A. Antitumorigenic Properties of Omega-3 Endocannabinoid Epoxides. J. Med. Chem. 2018, 61, 5569–5579. [Google Scholar] [CrossRef]
- Yang, L.; Li, F.F.; Han, Y.C.; Jia, B.; Ding, Y. Cannabinoid receptor CB2 is involved in tetrahydrocannabinol-induced anti-inflammation against lipopolysaccharide in MG-63 cells. Mediat. Inflamm. 2015, 2015, 362126. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Fraguas-Sanchez, A.I.; Martin-Sabroso, C.; Torres-Suarez, A.I. Insights into the effects of the endocannabinoid system in cancer: A review. Br. J. Pharmacol. 2018, 175, 2566–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [Green Version]
- Niu, F.; Zhao, S.; Xu, C.Y.; Sha, H.; Bi, G.B.; Chen, L.; Ye, L.; Gong, P.; Nie, T.H. Potentiation of the antitumor activity of adriamycin against osteosarcoma by cannabinoid WIN-55,212-2. Oncol. Lett. 2015, 10, 2415–2421. [Google Scholar] [CrossRef] [Green Version]
- Stampanoni Bassi, M.; Gentile, A.; Iezzi, E.; Zagaglia, S.; Musella, A.; Simonelli, I.; Gilio, L.; Furlan, R.; Finardi, A.; Marfia, G.A.; et al. Transient Receptor Potential Vanilloid 1 Modulates Central Inflammation in Multiple Sclerosis. Front. Neurol. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Di Marzo, V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium 2009, 45, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Templeton, D.M. Cadmium activates CaMK-II and initiates CaMK-II-dependent apoptosis in mesangial cells. FEBS Lett. 2007, 581, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Bellini, G.; Luongo, L.; Torella, M.; Mancusi, S.; De Petrocellis, L.; Petrosino, S.; Siniscalco, D.; Orlando, P.; Scafuro, M.; et al. The endovanilloid/endocannabinoid system: A new potential target for osteoporosis therapy. Bone 2011, 48, 997–1007. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Torella, M.; Tortora, C.; Manzo, I.; Giordano, C.; Guida, F.; Luongo, L.; Papale, F.; Rosso, F.; et al. The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Br. J. Pharmacol. 2014, 171, 2621–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Siniscalco, D.; Luongo, L.; De Petrocellis, L.; Bellini, G.; Petrosino, S.; Torella, M.; Santoro, C.; Nobili, B.; Perrotta, S.; et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009, 44, 476–484. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Andre, F.; Tesniere, A.; Kroemer, G. The anticancer immune response: Indispensable for therapeutic success? J. Clin. Investig. 2008, 118, 1991–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.; Li, B.; Wang, S.; Chen, T.; Ye, Z. Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma. Front. Immunol. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Erp, A.E.M.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.S.; van Houdt, L.; Gorris, M.A.J.; van Dam, L.S.; Mentzel, T.; Weidema, M.E.; Savci-Heijink, C.D.; Desar, I.M.E.; et al. Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8(+) lymphocytes in primary sarcomas is subtype dependent. Oncotarget 2017, 8, 71371–71384. [Google Scholar] [CrossRef] [Green Version]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [Green Version]
- Ando, K.; Mori, K.; Corradini, N.; Redini, F.; Heymann, D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expert Opin. Pharmacother. 2011, 12, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, P.A.; Chou, A.J. Muramyl tripeptide-phosphatidyl ethanolamine encapsulated in liposomes (L-MTP-PE) in the treatment of osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 307–321. [Google Scholar]
- Georgoudaki, A.M.; Prokopec, K.E.; Boura, V.F.; Hellqvist, E.; Sohn, S.; Ostling, J.; Dahan, R.; Harris, R.A.; Rantalainen, M.; Klevebring, D.; et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep. 2016, 15, 2000–2011. [Google Scholar] [CrossRef] [Green Version]
- Punzo, F.; Bellini, G.; Tortora, C.; Pinto, D.D.; Argenziano, M.; Pota, E.; Paola, A.D.; Martino, M.D.; Rossi, F. Mifamurtide and TAM-like macrophages: Effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget 2020, 11, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Grupp, S.A.; Verneris, M.; Sondel, P.M.; Cooper, L.J. Immunotherapy for pediatric cancer. Biol. Blood Marrow Transplant. 2008, 14 (Suppl. 1), 33–43. [Google Scholar] [CrossRef] [Green Version]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets for Bone and Soft-Tissue Sarcomas. Int. J. Mol. Sci. 2019, 20, 170. [Google Scholar] [CrossRef] [Green Version]
- Mehta, C.R.; Liu, L.; Theuer, C. An adaptive population enrichment phase III trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma (TAPPAS trial). Ann. Oncol. 2019, 30, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Barris, D.M.; Piperdi, S.; Kuo, V.; Everts, S.; Geller, D.; Houghton, P.; Kolb, E.A.; Hawthorne, T.; Gill, J.; et al. Targeting Glycoprotein NMB With Antibody-Drug Conjugate, Glembatumumab Vedotin, for the Treatment of Osteosarcoma. Pediatr. Blood Cancer 2016, 63, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.K.; Appel, N.; Labban, N.; Lussier, D.M.; Blattman, J.N.; Hingorani, P. Progress and opportunities for immune therapeutics in osteosarcoma. Immunotherapy 2016, 8, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.; Saleh, M.; Rose, A.A.; Siegel, P.M.; Hart, L.; Sirpal, S.; Jones, S.; Green, J.; Crowley, E.; Simantov, R.; et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Kolb, E.A.; Gorlick, R.; Billups, C.A.; Hawthorne, T.; Kurmasheva, R.T.; Houghton, P.J.; Smith, M.A. Initial testing (stage 1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical testing program. Pediatr. Blood Cancer 2014, 61, 1816–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachelot, T.; Ciruelos, E.; Schneeweiss, A.; Puglisi, F.; Peretz-Yablonski, T.; Bondarenko, I.; Paluch-Shimon, S.; Wardley, A.; Merot, J.L.; du Toit, Y.; et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann. Oncol. 2019, 30, 766–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Jensen, P.E.; Chen, X. Elimination of Osteosarcoma by Necroptosis with Graphene Oxide-Associated Anti-HER2 Antibodies. Int. J. Mol. Sci. 2019, 20, 4360. [Google Scholar] [CrossRef] [Green Version]
- Dore, R.K. The RANKL pathway and denosumab. Rheum. Dis. Clin. N. Am. 2011, 37, 433–452, vi–vii. [Google Scholar] [CrossRef]
- Errani, C.; Tsukamoto, S.; Mavrogenis, A.F. How safe and effective is denosumab for bone giant cell tumour? Int. Orthop. 2017, 41, 2397–2400. [Google Scholar] [CrossRef]
- Stadler, N.; Fingernagel, T.; Hofstaetter, S.G.; Trieb, K. A recurrent giant cell tumor of bone treated with denosumab. Clin. Pract. 2015, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Branstetter, D.; Rohrbach, K.; Huang, L.Y.; Soriano, R.; Tometsko, M.; Blake, M.; Jacob, A.P.; Dougall, W.C. RANK and RANK ligand expression in primary human osteosarcoma. J. Bone Oncol. 2015, 4, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [CrossRef]
- Chen, Y.; Di Grappa, M.A.; Molyneux, S.D.; McKee, T.D.; Waterhouse, P.; Penninger, J.M.; Khokha, R. RANKL blockade prevents and treats aggressive osteosarcomas. Sci. Transl. Med. 2015, 7, 317ra197. [Google Scholar] [CrossRef] [PubMed]
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Bago-Horvath, Z.; Schmid, K.; Rossler, F.; Nagy-Bojarszky, K.; Funovics, P.; Sulzbacher, I. Impact of RANK signalling on survival and chemotherapy response in osteosarcoma. Pathology 2014, 46, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Even-Chena, T.; Katzavian, G.; Lehmann, D.; Dagan, A.; Mechoulam, R. Gamma-irradiation enhances apoptosis induced by cannabidiol, a non-psychotropic cannabinoid, in cultured HL-60 myeloblastic leukemia cells. Leuk. Lymphoma 2003, 44, 1767–1773. [Google Scholar] [CrossRef]
- Gibiansky, L.; Sutjandra, L.; Doshi, S.; Zheng, J.; Sohn, W.; Peterson, M.C.; Jang, G.R.; Chow, A.T.; Perez-Ruixo, J.J. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin. Pharmacokinet. 2012, 51, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Botter, S.M.; Neri, D.; Fuchs, B. Recent advances in osteosarcoma. Curr. Opin. Pharmacol. 2014, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A. Osteosarcoma in Korean children and adolescents. Korean J. Pediatr. 2015, 58, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Punzo, F.; Tortora, C.; Argenziano, M.; Pinto, D.D.; Pota, E.; Martino, M.D.; Paola, A.D.; Rossi, F. Can Denosumab be used in combination with Doxorubicin in Osteosarcoma? Oncotarget 2020, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.S.; Shah, A.; Stanworth, S.J.; Frise, C.J.; Spiby, H.; Lax, S.J.; Murray, J.; Klein, A.A. The effect of iron deficiency and anaemia on women’s health. Anaesthesia 2021, 76 (Suppl. 4), 84–95. [Google Scholar] [CrossRef]
- Litton, E.; Lim, J. Iron Metabolism: An Emerging Therapeutic Target in Critical Illness. Crit. Care 2019, 23, 81. [Google Scholar] [CrossRef] [Green Version]
- Ni, S.; Kuang, Y.; Yuan, Y.; Yu, B. Mitochondrion-mediated iron accumulation promotes carcinogenesis and Warburg effect through reactive oxygen species in osteosarcoma. Cancer Cell Int. 2020, 20, 399. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal Med. 2007, 12, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Zhou, Y.; He, H.; Chen, W.; Lenahan, C.; Li, X.; Deng, Y.; Shao, A.; Huang, J. Crosstalk between Macrophages, T Cells, and Iron Metabolism in Tumor Microenvironment. Oxid. Med. Cell. Longev. 2021, 2021, 8865791. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef]
- Forciniti, S.; Greco, L.; Grizzi, F.; Malesci, A.; Laghi, L. Iron Metabolism in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2257. [Google Scholar] [CrossRef] [Green Version]
- Gammella, E.; Buratti, P.; Cairo, G.; Recalcati, S. The transferrin receptor: The cellular iron gate. Metallomics 2017, 9, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.Z. Cellular Iron Metabolism and Regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar]
- Argenziano, M.; Di Paola, A.; Tortora, C.; Di Pinto, D.; Pota, E.; Di Martino, M.; Perrotta, S.; Rossi, F.; Punzo, F. Effects of Iron Chelation in Osteosarcoma. Curr. Cancer Drug Targets 2021, 21, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Tomat, E.; Brune, B. Iron as a Central Player and Promising Target in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2018, 20, 95. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Rivella, S. Cancer cells with irons in the fire. Free Radic. Biol. Med. 2015, 79, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Lv, H.; Zhao, B.; Zhou, L.; Wang, S.; Luo, J.; Liu, J.; Shang, P. Iron and leukemia: New insights for future treatments. J. Exp. Clin. Cancer Res. 2019, 38, 406. [Google Scholar] [CrossRef] [Green Version]
- Le, N.T.; Richardson, D.R. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim. Biophys. Acta 2002, 1603, 31–46. [Google Scholar] [CrossRef]
- Raza, M.; Chakraborty, S.; Choudhury, M.; Ghosh, P.C.; Nag, A. Cellular iron homeostasis and therapeutic implications of iron chelators in cancer. Curr. Pharm. Biotechnol. 2014, 15, 1125–1140. [Google Scholar] [CrossRef]
- Greene, C.J.; Attwood, K.; Sharma, N.J.; Gross, K.W.; Smith, G.J.; Xu, B.; Kauffman, E.C. Transferrin receptor 1 upregulation in primary tumor and downregulation in benign kidney is associated with progression and mortality in renal cell carcinoma patients. Oncotarget 2017, 8, 107052–107075. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, A.; Deaglio, S.; Maldi, E.; Cassoni, P.; Malavasi, F.; Testa, U. TfR2 expression in human colon carcinomas. Blood Cells Mol. Dis. 2009, 43, 243–249. [Google Scholar] [CrossRef]
- Calzolari, A.; Finisguerra, V.; Oliviero, I.; Deaglio, S.; Mariani, G.; Malavasi, F.; Testa, U. Regulation of transferrin receptor 2 in human cancer cell lines. Blood Cells Mol. Dis. 2009, 42, 5–13. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Han, B.; Connor, J.R. Ferritin stimulates breast cancer cells through an iron-independent mechanism and is localized within tumor-associated macrophages. Breast Cancer Res. Treat. 2013, 137, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.; De Bortoli, M.; Ciniselli, C.M.; Vaghi, E.; Caccia, D.; Garrisi, V.; Pizzamiglio, S.; Veneroni, S.; Bonini, C.; Agresti, R.; et al. Hepcidin and ferritin blood level as noninvasive tools for predicting breast cancer. Ann. Oncol. 2014, 25, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, Z.K.; Miller, L.D.; Wang, W.; D’Agostino, R., Jr.; Kute, T.; Willingham, M.C.; Hatcher, H.; Tesfay, L.; Sui, G.; Di, X.; et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010, 2, 43ra56. [Google Scholar] [CrossRef]
- Thevenod, F. Iron and Its Role in Cancer Defense: A Double-Edged Sword. Met. Ions Life Sci. 2018, 18, 437–468. [Google Scholar] [CrossRef]
- Marques, O.; Porto, G.; Rema, A.; Faria, F.; Cruz Paula, A.; Gomez-Lazaro, M.; Silva, P.; Martins da Silva, B.; Lopes, C. Local iron homeostasis in the breast ductal carcinoma microenvironment. BMC Cancer 2016, 16, 187. [Google Scholar] [CrossRef] [Green Version]
- Recalcati, S.; Locati, M.; Marini, A.; Santambrogio, P.; Zaninotto, F.; De Pizzol, M.; Zammataro, L.; Girelli, D.; Cairo, G. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 2010, 40, 824–835. [Google Scholar] [CrossRef]
- Leftin, A.; Zhao, H.; Turkekul, M.; de Stanchina, E.; Manova, K.; Koutcher, J.A. Iron deposition is associated with differential macrophage infiltration and therapeutic response to iron chelation in prostate cancer. Sci. Rep. 2017, 7, 11632. [Google Scholar] [CrossRef]
- Mertens, C.; Akam, E.A.; Rehwald, C.; Brune, B.; Tomat, E.; Jung, M. Intracellular Iron Chelation Modulates the Macrophage Iron Phenotype with Consequences on Tumor Progression. PLoS ONE 2016, 11, e0166164. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Tolba, O.A. Iron chelation monotherapy in transfusion-dependent beta-thalassemia major patients: A comparative study of deferasirox and deferoxamine. Electron. Physician 2016, 8, 2425–2431. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.L.; Lee, D.H.; Na, Y.J.; Kim, B.R.; Jeong, Y.A.; Lee, S.I.; Kang, S.; Joung, S.Y.; Lee, S.Y.; Oh, S.C.; et al. Iron chelator-induced apoptosis via the ER stress pathway in gastric cancer cells. Tumour. Biol. 2016, 37, 9709–9719. [Google Scholar] [CrossRef] [PubMed]
- Linden, T.; Wenger, R.H. Iron chelation, angiogenesis and tumor therapy. Int. J. Cancer 2003, 106, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, J.S.; Won, Y.W.; Uhm, J.; Park, B.B.; Lee, Y.Y. The potential of deferasirox as a novel therapeutic modality in gastric cancer. World J. Surg. Oncol. 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, S.J.; Obeidy, P.; Lovejoy, D.B.; Bedford, M.; Nichols, L.; Chadwick, C.; Tucker, O.; Lui, G.Y.; Kalinowski, D.S.; Jansson, P.J.; et al. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br. J. Pharmacol. 2013, 168, 1316–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, G.Y.; Obeidy, P.; Ford, S.J.; Tselepis, C.; Sharp, D.M.; Jansson, P.J.; Kalinowski, D.S.; Kovacevic, Z.; Lovejoy, D.B.; Richardson, D.R. The iron chelator, deferasirox, as a novel strategy for cancer treatment: Oral activity against human lung tumor xenografts and molecular mechanism of action. Mol. Pharmacol. 2013, 83, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punzo, F.; Tortora, C.; Argenziano, M.; Casale, M.; Perrotta, S.; Rossi, F. Iron chelating properties of Eltrombopag: Investigating its role in thalassemia-induced osteoporosis. PLoS ONE 2018, 13, e0208102. [Google Scholar]
- Vlachodimitropoulou, E.; Chen, Y.L.; Garbowski, M.; Koonyosying, P.; Psaila, B.; Sola-Visner, M.; Cooper, N.; Hider, R.; Porter, J. Eltrombopag: A powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood 2017, 130, 1923–1933. [Google Scholar] [CrossRef]
- Kalota, A.; Selak, M.A.; Garcia-Cid, L.A.; Carroll, M. Eltrombopag modulates reactive oxygen species and decreases acute myeloid leukemia cell survival. PLoS ONE 2015, 10, e0126691. [Google Scholar] [CrossRef]
- Roth, M.; Will, B.; Simkin, G.; Narayanagari, S.; Barreyro, L.; Bartholdy, B.; Tamari, R.; Mitsiades, C.S.; Verma, A.; Steidl, U. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood 2012, 120, 386–394. [Google Scholar] [CrossRef]
- Shi, M.; Xu, F.; Yang, X.; Bai, Y.; Niu, J.; Drokow, E.K.; Chen, M.; Chen, Y.; Sun, K. The synergistic antileukemic effects of eltrombopag and decitabine in myeloid leukemia cells. Cancer Manag. Res. 2019, 11, 8229–8238. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, T.; Murata, S.; Zheng, Y.W.; Iwasaki, K.; Kohno, K.; Fukunaga, K.; Ohkohchi, N. The Eltrombopag antitumor effect on hepatocellular carcinoma. Int. J. Oncol. 2015, 47, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zheng, X.; Shou, K.; Niu, Y.; Jian, C.; Zhao, Y.; Yi, W.; Hu, X.; Yu, A. The iron chelator Dp44mT suppresses osteosarcoma’s proliferation, invasion and migration: In vitro and in vivo. Am. J. Transl. Res. 2016, 8, 5370–5385. [Google Scholar]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Psaila, B.; Lyden, D. Bone marrow cells in the ‘pre-metastatic niche’: Within bone and beyond. Cancer Metastasis Rev. 2006, 25, 521–529. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Senerchia, A.A.; Macedo, C.R.; Ferman, S.; Scopinaro, M.; Cacciavillano, W.; Boldrini, E.; Lins de Moraes, V.L.; Rey, G.; de Oliveira, C.T.; Castillo, L.; et al. Results of a randomized, prospective clinical trial evaluating metronomic chemotherapy in nonmetastatic patients with high-grade, operable osteosarcomas of the extremities: A report from the Latin American Group of Osteosarcoma Treatment. Cancer 2017, 123, 1003–1010. [Google Scholar] [CrossRef]
- Li, J.P.; Liu, L.H.; Li, J.; Chen, Y.; Jiang, X.W.; Ouyang, Y.R.; Liu, Y.Q.; Zhong, H.; Li, H.; Xiao, T. Microarray expression profile of long noncoding RNAs in human osteosarcoma. Biochem. Biophys. Res. Commun. 2013, 433, 200–206. [Google Scholar] [CrossRef]
- Chan, L.H.; Wang, W.; Yeung, W.; Deng, Y.; Yuan, P.; Mak, K.K. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 2014, 33, 4857–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Liang, G.; Yuan, B.; Yang, C.; Gao, R.; Zhou, X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour. Biol. 2015, 36, 1477–1486. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, F.; Fei, Z.; Zhao, J.; Liang, Y.; Pan, W.; Liu, X.; Zheng, D. Genetic variants of lncRNA HOTAIR contribute to the risk of osteosarcoma. Oncotarget 2016, 7, 19928–19934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignochino, Y.; Grignani, G.; Cavalloni, G.; Motta, M.; Tapparo, M.; Bruno, S.; Bottos, A.; Gammaitoni, L.; Migliardi, G.; Camussi, G.; et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol. Cancer 2009, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Niu, X.; Yao, W. Receptor Tyrosine Kinases in Osteosarcoma Treatment: Which Is the Key Target? Front. Oncol. 2020, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Dileo, P.; Asaftei, S.D.; D’Ambrosio, L.; Pignochino, Y.; Mercuri, M.; Picci, P.; Fagioli, F.; Casali, P.G.; et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann. Oncol. 2012, 23, 508–516. [Google Scholar] [CrossRef]
- Kiyuna, T.; Tome, Y.; Miyake, K.; Murakami, T.; Oshiro, H.; Igarashi, K.; Kawaguchi, K.; Hsu, J.; Singh, M.; Li, Y.; et al. Eribulin Suppressed Cisplatinum- and Doxorubicin-resistant Recurrent Lung Metastatic Osteosarcoma in a Patient-derived Orthotopic Xenograft Mouse Model. Anticancer Res. 2019, 39, 4775–4779. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.V. GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J. Hematol. Oncol. 2020, 13, 172. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, W.H.; Lee, J.A.; Kim, D.H.; Seo, J.H.; Lim, J.S. Bone mineral density change during adjuvant chemotherapy in pediatric osteosarcoma. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 150–154. [Google Scholar] [CrossRef]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurdan, T.; Hatice Mine, C.; Emel, U.; Handan, D.; Gulsah, T.; Incesoy Ozdemir, S.; Omer, K.; Yasin, Y.; Yusuf, Y.; Basarir, K.; et al. Late effects of osteosarcoma and its treatment in pediatric patients: A single-center experience. J. BUON 2021, 26, 1102–1110. [Google Scholar] [PubMed]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [PubMed]
- Jorgensen, A.R.; Jorgensen, P.H.; Jul Kiil, B.; Stilling, M. Bone mineral density changes in a free vascularised fibular graft in the distal femoral bone after osteosarcoma in a 10-year-old boy: A 7-year follow-up. BMJ Case Rep. 2021, 14, e236097. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-alpha and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef]
- Yang, S.; Wu, C.; Wang, L.; Shan, D.; Chen, B. Pretreatment inflammatory indexes as prognostic predictors for survival in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2020, 13, 515–524. [Google Scholar]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Benner, B.; Scarberry, L.; Suarez-Kelly, L.P.; Duggan, M.C.; Campbell, A.R.; Smith, E.; Lapurga, G.; Jiang, K.; Butchar, J.P.; Tridandapani, S.; et al. Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J. Immunother. Cancer 2019, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Fribley, A.; Zeng, Q.; Wang, C.Y. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol. Cell Biol. 2004, 24, 9695–9704. [Google Scholar] [CrossRef] [Green Version]
- Saez, I.; Vilchez, D. The Mechanistic Links Between Proteasome Activity, Aging and Age-related Diseases. Curr. Genom. 2014, 15, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.R.C.; Abdul-Majeed, S.; Cael, B.; Barta, S.K. Clinical Pharmacokinetics and Pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2019, 58, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M. Denosumab: An Emerging Therapy in Pediatric Bone Disorders. Curr. Osteoporos. Rep. 2017, 15, 283–292. [Google Scholar] [CrossRef] [PubMed]




| Novel Therapeutic Strategies | |
|---|---|
| Type of Drugs | Examples |
| Proteosome Inhibitors | MLN2238, Bortezomib |
| Endocannabinoid/Endovanilloid Receptors Agonists | JWH-133, RTX |
| Immune-Modulators | Mifamurtide |
| Iron Chelators | Deferasirox, Deferoxamine, Dp44mT, Eltrombopag |
| Monoclonal Antibodies | Glembatumumab-vedotin, Trastuzumab |
| Tyrosine Kinase Inhibitors (TKIs) | Sorafenib, Eribulin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argenziano, M.; Tortora, C.; Pota, E.; Di Paola, A.; Di Martino, M.; Di Leva, C.; Di Pinto, D.; Rossi, F. Osteosarcoma in Children: Not Only Chemotherapy. Pharmaceuticals 2021, 14, 923. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090923
Argenziano M, Tortora C, Pota E, Di Paola A, Di Martino M, Di Leva C, Di Pinto D, Rossi F. Osteosarcoma in Children: Not Only Chemotherapy. Pharmaceuticals. 2021; 14(9):923. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090923
Chicago/Turabian StyleArgenziano, Maura, Chiara Tortora, Elvira Pota, Alessandra Di Paola, Martina Di Martino, Caterina Di Leva, Daniela Di Pinto, and Francesca Rossi. 2021. "Osteosarcoma in Children: Not Only Chemotherapy" Pharmaceuticals 14, no. 9: 923. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14090923