The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
:1. Introduction
2. Results
2.1. Literature Search and Study Selection
2.2. Risk of Bias Assessment
2.3. Study Characteristics
2.3.1. Nabilone
2.3.2. Dronabinol
2.4. Outcomes
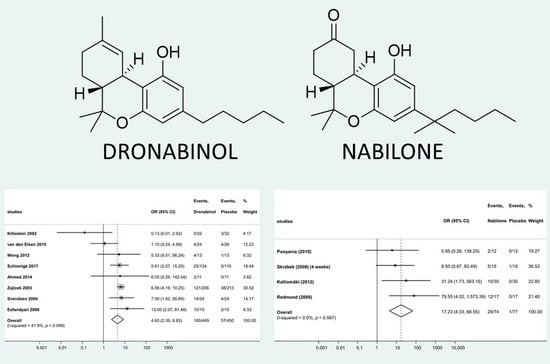
2.4.1. Quantitative Analysis—Nabilone
2.4.2. Quantitative Analysis—Dronabinol
2.5. Qualitative Analysis of Excluded Studies
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Eligibility Criteria
4.3. Study Selection
4.4. Data Extraction and Synthesis of the Results
4.5. Risk of Bias
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Todaro, B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J. Natl. Compr. Cancer Netw. 2012, 10, 487–492. [Google Scholar] [CrossRef] [PubMed]
- MARINOL® (Dronabinol). NDA 18-651/S-021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018651s021lbl.pdf (accessed on 11 December 2021).
- CESAMET (Nabilone) Capsules. NDA 18-677/S-011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf (accessed on 11 December 2021).
- Wallach, J. Medicinal Cannabis: An overview for health-care providers. Remington 2021, 75-101, 75–101. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen, A.C.; Staats, P.G.; Gorter, R.W.; Uitdehaag, B.M.; Polman, C.H. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002, 58, 1404–1407. [Google Scholar] [CrossRef]
- Malik, Z.; Bayman, L.; Valestin, J.; Rizvi-Toner, A.; Hashmi, S.; Schey, R. Dronabinol increases pain threshold in patients with functional chest pain: A pilot double-blind placebo-controlled trial. Dis. Esophagus 2016, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, T.; Camilleri, M.; Ferber, I.; Burton, D.; Baxter, K.; Zinsmeister, A.R. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: A randomized, placebo-controlled study. Neurogastroenterol. Motil. 2006, 18, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Camilleri, M.; Eckert, D.; Carlson, P.; Ryks, M.; Burton, D.; Zinsmeister, A.R. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol. Motil. 2012, 24, 358-e169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redmond, W.J.; Goffaux, P.; Potvin, S.; Marchand, S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr. Med Res. Opin. 2008, 24, 1017–1024. [Google Scholar] [CrossRef]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Haydn, T.; Müller, J.; Brenneis, C.; Berger, T.; Poewe, W.; Schelosky, L.D. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: A double-blind placebo-controlled cross-over trial. J. Neurol. 2006, 253, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can. J. Anesth. 2006, 53, 769–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliomäki, J.; Philipp, A.; Baxendale, J.; Annas, P.; Karlsten, R.; Segerdahl, M. Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2012, 39, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Elsen, G.A.V.D.; Ahmed, A.I.; Verkes, R.-J.; Kramers, C.; Feuth, T.; Rosenberg, P.B.; Van Der Marck, M.A.; Rikkert, M.G.O. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: A randomized controlled trial. Neurology 2015, 84, 2338–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003, 362, 1517–1526. [Google Scholar] [CrossRef]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004, 329, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsen, G.A.V.D.; Ahmed, A.I.; Verkes, R.-J.; Feuth, T.; van der Marck, M.A.; Rikkert, M.G.O. Tetrahydrocannabinol in behavioral disturbances in dementia: A crossover randomized controlled trial. Am. J. Geriatr. Psychiatry 2015, 23, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; Elsen, G.A.V.D.; Colbers, A.; van der Marck, M.A.; Burger, D.M.; Feuth, T.B.; Rikkert, M.G.O.; Kramers, C. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: A randomized controlled trial. Eur. Neuropsychopharmacol. 2014, 24, 1475–1482. [Google Scholar] [CrossRef]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.P.L.; Kiss, A.; Black, S.E.; Lanctôt, K.L. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Pooyania, S.; Ethans, K.; Szturm, T.; Casey, A.; Perry, D. A Randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2010, 91, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Cano, M.G.; McManus, D.; Mallik, S.; Hobart, J. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Rocha, F.C.M.; Stéfano, S.C.; Haiek, R.D.C.; Oliveira, L.M.Q.R.; Da Silveira, D.X. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: Systematic review and meta-analysis. Eur. J. Cancer Care 2008, 17, 431–443. [Google Scholar] [CrossRef]
- Smith, L.A.; Azariah, F.; Lavender, V.T.; Stoner, N.S.; Bettiol, S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015, 2021, CD009464. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, E.; Zaki, P.; Raman, S.; Olson, R.; McFarlane, T.; DeAngelis, C.; Chan, S.; Pidduck, W.; Razvi, Y.; Bushehri, A.; et al. Radiation-induced nausea and vomiting: A comparison between MASCC/ESMO, ASCO, and NCCN antiemetic guidelines. Support. Care Cancer 2019, 27, 783–791. [Google Scholar] [CrossRef]
- Razvi, Y.; Chan, S.; McFarlane, T.; McKenzie, E.; Zaki, P.; DeAngelis, C.; Pidduck, W.; Bushehri, A.; Chow, E.; Jerzak, K.J. ASCO, NCCN, MASCC/ESMO: A comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 2018, 27, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Spartinou, A.; Nyktari, V.; Papaioannou, A. Granisetron: A review of pharmacokinetics and clinical experience in chemotherapy induced—Nausea and vomiting. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1289–1297. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]




| First Author, Year | Country | Study Drug | Posology | Duration | Enrolled Patients | Patients Who Have Completed the Trial | Mean Age [yrs (SD)] | Sex [M/F (N)] | Outcomes | Reported Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Hermann, 2019 | Canada | nabilone | 1–2 mg once a day | 14 weeks | 39 | 33 | placebo & active: 87 (10) | 30/9 | Efficacy and safety of nabilone for agitation with moderate to severe Alzheimer’s | Sedation (including lethargy, treatment limiting sedation, significant increase in NPS, myocardial infarction, bradycardia, rash, dizziness, lethargy |
| Kalliomäki 2012 | UK | nabilone | 1–3 mg | 7 weeks & 5 days | 30 | 24 | placebo & active: 29.3 (no data) | 30/0 | Effect of nabilone on capsaicin-induced pain and hyperalgesia and on other CNS biomarkers | Somnolence, Postural dizziness, Tachycardia, Bradycardia, Dizziness, Headache, Fatigue, Dry mouth |
| Pooyania, 2010 | Canada | nabilone | 0.5 mg once or bid | 10 weeks | 12 | 11 | placebo & active: 42.36 (no data) | 11/0 | Alleviation of spasticity in patients with spinal cord injury (SCI) | Ataxia, Drowsiness, Vertigo (mild), Lack of motivation, Headache, Asthenia, Dry mouth |
| Redmond, 2008 | Canada | nabilone | 0.5–1 mg | 3 visits with washout periods of at least one week | 20 | 17 | placebo & active: male: 22.5 (1.5) female: 23.2 (2.8) | 7/10 | Analgesic and antihyperalgesic properties of nabilone | Mild sedation, Euphoria, Feeling cold, Nausea, Dizziness, Headache, Increased appetite, Dry mouth |
| Skrabek, 2008 | Canada | nabilone | 0.5–1 mg bid | 4 weeks | 40 | 33 | placebo: 50.11 (5.96) active: 47.6 (9.13) | 37/3 | Benefit of nabilone in pain management and QoL improvement in patients with fibromyalgia | Euphoria, Depression, Psychological high, Dissociation, Nightmares, Decreased concentration, Ataxia, Confusion, Hallucination, Orthostatic hypotension, Tachycardia, Sensory disturbance, Drowsiness, Lightheaded, Vertigo, Headache, Dysphoria, Anorexia, Dry mouth |
| Wissel, 2006 | Austria/Germany | nabilone | 0.5 mg once or tid | 9 weeks | 13 | 11 | placebo & active: 44.85 (13.82) | 4/9 | Efficacy and safety of low dose nabilone in spasticity related pain | Dysphagia (slight), Drowsiness, Weakness in lower limbs (slight) |
| First Author, Year | Country | Study Drug | Posology | Duration | Enrolled Patients | Patients Who Have Completed the Trial | Mean Age [yrs (SD)] | Sex [M/F (N)] | Outcomes | Reported Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Malik, 2007 | USA | dronabinol | 5 mg bid for 4 weeks | 4 weeks | 19 | 13 | placebo: 42 (ND) active: 44 (ND) | 2/11 | Effect of dronabinol on pain threshold, frequency, and intensity in functional chest pain (FCP) | Loose stools, nausea, headache, fatigue |
| Schimrigk, 2017 | Germany | dronabinol | titration to daily doses 7.5–15.0 mg | 16 weeks | 240 | 169 | placebo: 47 (9.7) active: 48.4 (9.6) | 65/175 | Positive benefit–risk ratio of dronabinol in the treatment of neuropathic pain in MS patients | Insomnia, Nausea, Dizziness, Vertigo, Headache, Fatigue, Dry mouth |
| van den Elsen, 2015 | The Netherlands | dronabinol | 1.5 mg tid for 3 weeks | 3 weeks | 50 | 50 | placebo: 78 (7) active: 79 (8) | 25/25 | Efficacy and safety of THC in the treatment of dementia-related neuropsychiatric symptoms (NPS) | Delirium, Cognitive disorder, Euphoric mood, Bradykinesia, Somnolence, Agitation, Nasopharyngitis, Pneumonia, COPD, Back pain, Muscle weakness, Muscle spasms, Pain in extremity, Renal impairment, Urge incontinence, Dry eye, Eye hemorrhage, Miosis, Balance disorder, Chest pain, Skin disorder, not otherwise specified, Dizziness, Sensory loss, Restlessness, Aphasia, Apraxia, Headache, Fatigue, Malaise, Presyncope, Syncope, Decreased appetite, Increased gamma-glutamyl transferase, |
| Ahmed, 2014 | The Netherlands | dronabinol | 3–6.5 mg | 6 weeks | 12 | 11 | placebo & active: 72.1 (5) | 6/6 | Safety and tolerability effects of THC in elderly | Euphoria, Concentration problem, Visual hallucination, Relaxation, Dry eye, Blurred vision, Nausea, Coordination disturbance, Drowsiness, Dizziness, Headache, Malaise, Dry mouth |
| Wong, 2012 | USA | dronabinol | 2.5 or 5 mg bid | 2 days | 36 | 36 | placebo: 36.7 (3.1) active (2.5 mg): 47.7 (7.9) active (5 mg): 42.3 (4.5) | 2/34 | Gut transit in IBS-D and dronabinol’ transit effect | “Loopy”, foggy thinking, Hot flushes, Drowsiness/Tiredness, Dizziness/Light-headedness, Headache |
| Brisbois, 2011 | Canada | dronabinol | 2.5 mg bid (patients had the option to increase their drug dose to a maximum of 20 mg/day) | 3 weeks & 1 day | 46 | 21 | placebo: 65.5 (8) active: 67 (10.9) | 12/9 | Effects of THC on chemosensory perception | Confusion, Seizure, Troubles sleeping, Pneumonia, Thrush, Stomach cramps, Bowel obstruction/constipation, Diarrhea, Vaginal discharge, Unsteady feet, Shortness of breath/fluid on lungs, Nausea/Vomiting, Hives/Rash, Fever, Headache, Pain, Tired/Drowsy, Oedema, Low blood count |
| Esfandyari, 2006 | USA | dronabinol | 5–7.5 mg bid | 2 days | 30 | 30 (27) (3 patients did not complete the study; however, their missing data is included in the ITT analysis) | placebo: 29 (1) active: 26 (2) | 14/16 | Effect of dronabinol of gastrointestinal transit and postprandial satiation | Excitement, Euphoria/Relaxed, Disturbed mental concentration, Nausea, Numbness, Flushing, Drowsiness, Dizziness/Light-headedness, Headache, Vasovagal, Dry mouth |
| Svendsen, 2004 | Denmark | dronabinol | titration to 5 mg bid | 3 weeks | 24 | 24 | placebo & active: 50 (median) | 10/14 | Effect of dronabinol on central neuropathic pain in MS patients | Euphoria, Feeling of drunkenness, Speech disorders, Hyperactivity, Nervousness, Aggravated MS, Migraine, Sleep difficulty, Upper airway infection, Muscle weakness, Myalgia, Hot flushes, Diplopia, Balance difficulty, Palpitations, Abdominal pain, Nausea, Drowsiness, Dizziness, Fever, Headache, Fatigue, Anorexia, Weight decrease, Dry mouth, Chills |
| Zajicek, 2003 | UK | dronabinol | 2.5 mg | 15 weeks | 419 | 404 | placebo: 50.9 (7.6) active: 50.2 (8.2) | 141/278 | Effect of cannabinoids on spasticity and other symptoms in MS patients | Bladder, Depression of anxiety, Dizzy of light-headedness, Dry mouth, Gastrointestinal tract, Improvement in symptoms, Infection, Miscellaneous, Numbness of paresthesia, Pain, Sleep, Spasms of stiffness, Tremor of lack of coordination, Vision, Weakness of reduced mobility |
| Killestein, 2002 | The Netherlands | dronabinol | 2.5–5 mg bid | 4 weeks | 16 | 16 | placebo & active: 46 (7.9) | no data | Efficacy, safety, and tolerability effects of THC in MS patients | Emotional lability, Ataxia, Somnolence, Increased spasticity, Dizziness, Headache, Dry mouth, Other |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajtel, Á.; Kiss, T.; Tóth, B.; Kiss, S.; Hegyi, P.; Vörhendi, N.; Csupor-Löffler, B.; Gede, N.; Hohmann, J.; Csupor, D. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals 2022, 15, 100. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15010100
Bajtel Á, Kiss T, Tóth B, Kiss S, Hegyi P, Vörhendi N, Csupor-Löffler B, Gede N, Hohmann J, Csupor D. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals. 2022; 15(1):100. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15010100
Chicago/Turabian StyleBajtel, Ákos, Tivadar Kiss, Barbara Tóth, Szabolcs Kiss, Péter Hegyi, Nóra Vörhendi, Boglárka Csupor-Löffler, Noémi Gede, Judit Hohmann, and Dezső Csupor. 2022. "The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials" Pharmaceuticals 15, no. 1: 100. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15010100