Extensively Drug-Resistant Tuberculosis: A Sign of the Times and an Impetus for Antimicrobial Discovery
Abstract
:1. Introduction
2. Global Incidence, Prevalence, and Mortality of TB
| 2000 | 2006 | 2007 | 2008 | |
|---|---|---|---|---|
| Incidencea | 8.3 million | 9.24 million | 9.27 million | 9.4 million |
| Prevalenceb | 16.6 millionc | 13.9 million | 13.7 million | 11.1 million |
| Deaths (total) | 1.8 million | 1.7 million | 1.7 million | 1.8 million |
| HIV-negative | 1.6 million | 1.5 million | 1.3 million | 1.3 million |
| HIV-positive | 0.226 million | 0.231 million | 0.456 milliond | 0.52 milliond |
3. Recommended TB Treatment and the Current Arsenal of TB Antibiotics
| First-line antibiotics | Antibiotic class/structure | Delivery route | Activity | Mechanism of action | Genes and gene products associated with resistance |
|---|---|---|---|---|---|
| Isoniazid | Pyridine hydrazide | Oral | Bactericidal | Inhibits mycolic acid (cell wall) synthesis | katG; catalase-peroxidase |
| inhA; enoyl-ACP reductase | |||||
| ndh; NADH dehydrogenase II | |||||
| Rifampin | Rifamycin | Oral | Bactericidal | Inhibits RNA synthesis | rpoB; β-subunit of RNA polymerase |
| Pyrazinamide | Nicotinamide analog | Oral | Bacteriostatic/ bactericidal | Disrupts cell membrane energetics and inhibits membrane transport | pncA; nicotinamidase/pyrazinamidase |
| Ethambutol | Ethylenediamine derivative | Oral | Bacteriostatic | Inhibits arabinogalactan (cell wall) synthesis | embCAB; arabinosyl transferase |
4. Emergence and Global Health Impact of XDR-TB
| Second-line antibiotics | Antibiotic class/structure | Delivery routea | Activity | Mechanism of action | Genes and gene products associated with resistance |
|---|---|---|---|---|---|
| Streptomycin | Aminoglycoside | IM injection | Bactericidal | Inhibits protein synthesis | rpsL; S12 ribosomal protein |
| rrs ; 16S rRNA | |||||
| Kanamycin/Amikacin | Aminoglycoside | IM injection | Bactericidal | Inhibits protein synthesis | rrs; 16S rRNA |
| Capreomycin | Polypeptide | IM injection | Bactericidal | Inhibits protein synthesis | rrs; 16S rRNA |
| tlyA ; putative rRNA methyltransferase | |||||
| Levofloxacin | Fluoroquinolone | Oral or IV | Bactericidal | Inhibits DNA replication | gyrA; DNA gyrase subunit A |
| Moxifloxacin | Fluoroquinolone | Oral or IV | Bactericidal | Inhibits DNA replication | gyrA; DNA gyrase subunit A |
| Gatifloxacin | Fluoroquinolone | Oral or IV | Bactericidal | Inhibits DNA replication | gyrA; DNA gyrase subunit A |
| Ethionamide | Thioamide | Oral | Bacteriostatic | Inhibits mycolic acid (cell wall) synthesis | inhA; enoyl-ACP reductase |
| etaA/ethA ; flavin monooxygenase | |||||
| Cycloserine | Isoxazolidinone | Oral | Bacteriostatic | Inhibits peptidoglycan (cell wall) synthesis | unknown (alrA; D-alanine racemase in Mycobacterium smegmatis) |
| Para-aminosalicylic acid | Salicyclic acid | Oral | Bacteriostatic | Inhibits folic acid synthesis | thyA; thymidylate synthase |
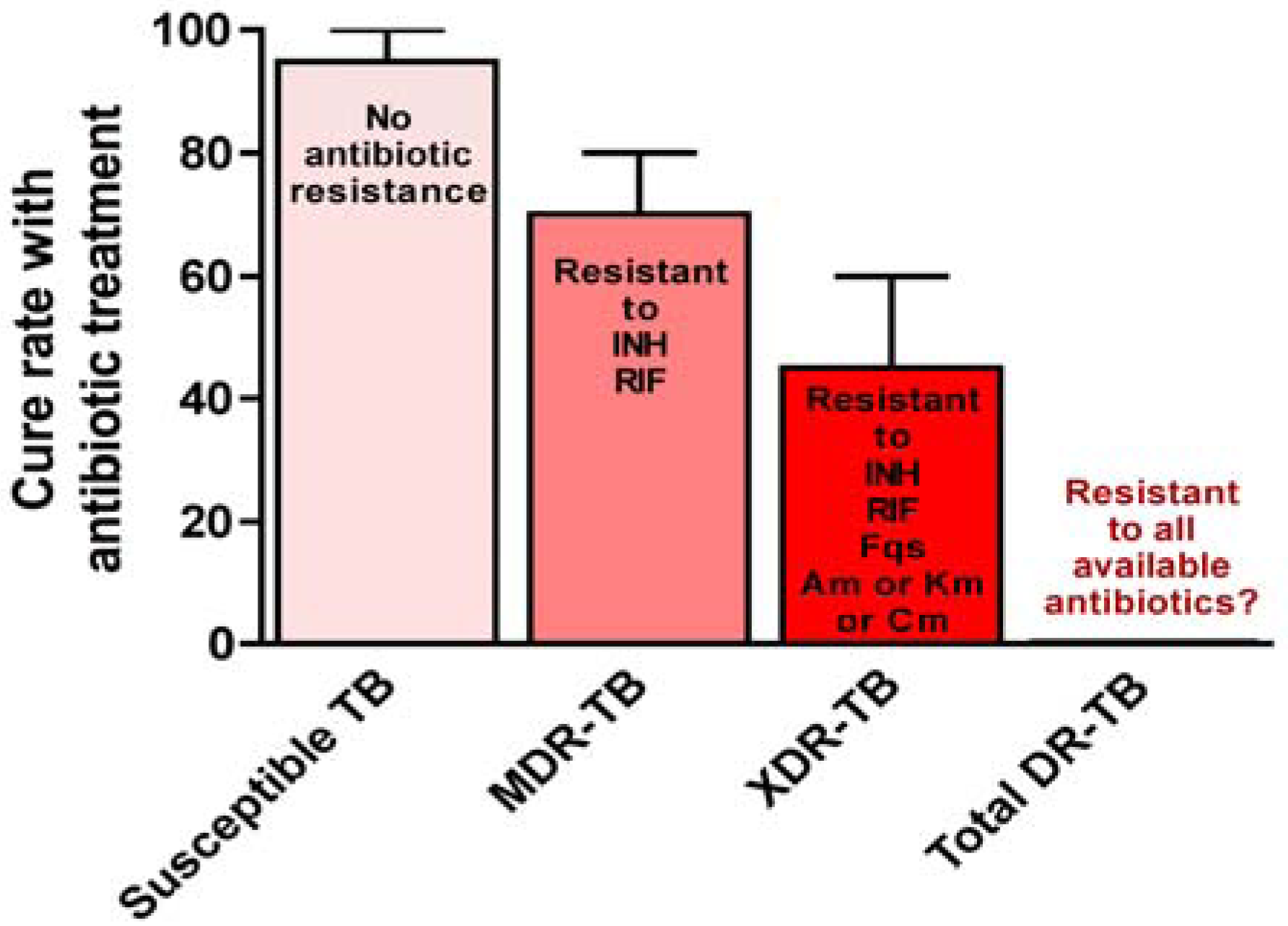
5. Genetic Basis of M. tuberculosis Antibiotic Resistance
6. Existing Treatment Regimens and Treatment Outcomes for XDR-TB
| Study | Study location | XDR-TB patients | HIV status | Treatment success rate |
|---|---|---|---|---|
| Gandhi et al. [105] | KwaZulu Natal, Republic of South Africa | 53 | positive | 2% |
| Migliori et al. [102] | Europe (Estonia, Germany, Italy, and Russian Federation) | 64 | negative | 39% |
| Keshavjee et al. [62] | Tomsk, Russia | 29 | negative | 48% |
| Chan et al. [57] | United States | 10 | negative | 50% |
| Mitnick et al. [112] | Peru | 48 | negative | 60% |
| Kim et al. [63] | South Korea | 75 | negative | 29% |
7. The Inherent Need for New Anti-TB Drugs
8. Development of Novel Chemotherapeutic Anti-TB Compounds
| Drug | Class | Mechanism of action |
|---|---|---|
| TMC207 | Diarylquinoline | Inhibits ATP synthase and energy metabolism |
| PA-824 | Nitroimidazo-oxazine | Inhibits mycolic acid synthesis |
| OPC-67683 | Nitrodihydro-imidazooxazole | Inhibits mycolic acid synthesis |
9. Future Perspectives Regarding the Discovery of New Anti-TB Compounds
10. Concluding Remarks and Perspectives
Acknowledgements
References
- Daniel, T.M.; Bates, J.H.; Downes, K.A. History of Tuberculosis. In Tuberculosis: Pathogenesis, Protection, and Control; Bloom, B.R., Ed.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 13–24. [Google Scholar]
- Bloom, B.R.; Murray, C.J.L. Tuberculosis: commentary on a reemergent killer. Science 1992, 257, 1055–1064. [Google Scholar]
- Flynn, J.L.; Chan, J. Tuberculosis: latency and reactivation. Infect. Immun. 2001, 69, 4195–4201. [Google Scholar]
- Clark-Curtiss, J.E.; Haydel, S.E. Molecular genetics of Mycobacterium tuberculosis pathogenesis. Annu. Rev. Microbiol. 2003, 57, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Maartens, G.; Wilkinson, R.J. Tuberculosis. Lancet 2007, 370, 2030–2043. [Google Scholar]
- Crofton, J.; Mitchison, D.A. Streptomycin resistance in pulmonary tuberculosis. Brit. Med. J. 1948, 2, 1009–1015. [Google Scholar]
- Schraufnagel, D.E. Tuberculosis treatment for the beginning of the next century. Int. J. Tuberc. Lung Dis. 1999, 3, 651–662. [Google Scholar]
- Corpe, R.F.; Blalock, F.A. Alternating regimens of streptomycin-pyrazinamide, isoniazid-para-aminosalicylic acid at Battey State Hospital. Amer. Rev. Resp. Dis. 1964, 90, 262–265. [Google Scholar]
- Corpe, R.F.; Blalock, F.A. Multi-drug therapy including ethambutol in retreatment of pulmonary tuberculosis. Ann. N Y Acad. Sci. 1966, 135, 823–830. [Google Scholar]
- Centres, R. The Madras Experiment. Lancet 1961, 2, 532–533. [Google Scholar]
- Espinal, M.A. The global situation of MDR-TB. Tuberculosis 2003, 83, 44–51. [Google Scholar]
- World Health Organization, TB/HIV Fact Sheet 2009; World Health Organization: Geneva, Switzerland, 2009.
- Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide, 2000-2004. MMWR Recomm. Rep. 2006, 55, 301–305.
- Raviglione, M.C.; Smith, I. XDR tuberculosis-implications for global public health. New Engl. J. Med. 2007, 356, 656–659. [Google Scholar]
- World Health Organization, Tuberculosis, Fact sheet No. 104; World Health Organization: Geneva, Switzerland, 2010.
- World Health Organization, Global Tuberculosis Control: A Short Update to the 2009 Report; World Health Organization: Geneva, Switzerland, 2009.
- Raviglione, M.C.; Uplekar, M.W. WHO's new Stop TB Strategy. Lancet 2006, 367, 952–955. [Google Scholar]
- World Health Organization, The Global Plan to Stop TB: 2006-2015; World Health Organization: Geneva, Switzerland, 2006.
- World Health Organization, Global Tuberculosis Control-Epidemiology, Strategy, Financing; World Health Organization: Geneva, Switzerland, 2009.
- Dye, C. Global epidemiology of tuberculosis. Lancet 2006, 367, 938–940. [Google Scholar]
- Dye, C.; Scheele, S.; Dolin, P.; Pathania, V.; Raviglione, M.C. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. JAMA 1999, 282, 677–686. [Google Scholar]
- Corbett, E.L.; Watt, C.J.; Walker, N.; Maher, D.; Williams, B.G.; Raviglione, M.C.; Dye, C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003, 163, 1009–1021. [Google Scholar]
- World Health Organization, Global Tuberculosis Control-Epidemiology, Strategy, Financing; World Health Organization: Geneva, Switzerland, 2007.
- Chaisson, R.E.; Martinson, N.A. Tuberculosis in Africa-Combating an HIV-driven crisis. N. Engl. J. Med. 2008, 358, 1089–1092. [Google Scholar]
- Ansari, N.A.; Kombe, A.H.; Kenyon, T.A.; Hone, N.M.; Tappero, J.W.; Nyirenda, S.T.; Binkin, N.J.; Lucas, S.B. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997-1998. Int. J. Tuberc. Lung Dis. 2002, 6, 55–63. [Google Scholar]
- World Health Organization, Global Tuberculosis Control-Epidemiology, Strategy, Financing; World Health Organization: Geneva, Switzerland, 2008.
- World Health Organization, Treatment of Tuberculosis Guidelines, 4th ed; World Health Organization: Geneva, Switzerland, 2010.
- Centers for Disease Control and Prevention. Treatment of tuberculosis: American Thoracic Society, CDC, and Infectious Diseases Society of America. MMWR Recomm. Rep. 2003, 52, 1–77.
- Kochi, A. Tuberculosis control—is DOTS the health breakthrough of the 1990s? World Health Forum 1997, 18, 225–247. [Google Scholar] [PubMed]
- World Health Organization, An Expanded DOTS Framework for Effective Tuberculosis Control; WHO: Geneva, Switzerland, 2002.
- World Health Organization. An expanded framework for effective tuberculosis control. Int. J. Tuberc. Lung Dis. 2002, 6, 378–388. [PubMed]
- Dye, C.; Watt, C.J.; Bleed, D.M.; Hosseini, S.M.; Raviglione, M.C. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA 2005, 293, 2767–2775. [Google Scholar] [PubMed]
- Jindani, A.; Aber, V.R.; Edwards, E.A.; Mitchison, D.A. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 1980, 121, 939–949. [Google Scholar]
- Hafner, R.; Cohn, J.A.; Wright, D.J.; Dunlap, N.E.; Egorin, M.J.; Enama, M.E.; Muth, K.; Peloquin, C.A.; Mor, N.; Heifets, L.B. Early bactericidal activity of isoniazid in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 1997, 156, 918–923. [Google Scholar]
- Dickinson, J.M.; Mitchison, D.A. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 1981, 159, 1580–1584. [Google Scholar]
- Girling, D.J. The role of pyrazinamide in primary chemotherapy for pulmonary tuberculosis. Tubercle 1984, 65, 1–4. [Google Scholar]
- Frieden, T.; Espinal, M. What is the Therapeutic Effect and What is the Toxicity of Antituberculosis Drugs? In Toman's Tuberculosis: Case Detection, Treatment and Monitoring: Questions and Answers, 2nd; Frieden, T., Ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 110–121. [Google Scholar]
- Dorman, S.E.; Chaisson, R.E. From magic bullets back to the Magic Mountain: the rise of extensively drug-resistant tuberculosis. Nat. Med. 2007, 13, 295–298. [Google Scholar]
- Frieden, T.; Sherman, L.; Maw, K.; Fujiwara, P.; Crawford, J.; Nivin, B.; Sharp, V.; Hewlett, D.J.; Brudney, K.; Alland, D.; Kreisworth, B. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 1996, 276, 1229–1235. [Google Scholar]
- Mukherjee, J.S.; Rich, M.L.; Socci, A.R.; Joseph, J.K.; Virú, F.A.; Shin, S.S.; Furin, J.J.; Becerra, M.C.; Barry, D.J.; Kim, J.Y.; et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 2004, 363, 474–481. [Google Scholar] [PubMed]
- World Health Organization, The Global Plan to Stop TB: 2001 to 2005; World Health Organization: Geneva, Switzerland, 2001.
- Gupta, R.; Cegielski, J.P.; Espinal, M.A.; Henkens, M.; Kim, J.Y.; Lambregts-van Weezenbeek, S.B.; Lee, J.W.; Raviglione, M.C.; Suarez, P.G.; Varaine, F. Increasing transparency in partnerships for health-introducing the Green Light Committee. Trop. Med. Int. Health 2002, 7, 970–976. [Google Scholar]
- Kir, A.; Tahaoglu, K.; Okur, E.; Hatipoglu, T. Role of surgery in multi-drug-resistant tuberculosis: results of 27 cases. Eur. J. Cardio-thorac. Surg. 1997, 12, 531–534. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, C.M.; Heu, J.P.; Song, S.D. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2002, 6, 143–149. [Google Scholar]
- Treasure, R.L.; Seaworth, B.J. Current role of surgery in Mycobacterium tuberculosis. Ann. Thorac. Surg. 1995, 59, 1405–1409. [Google Scholar] [PubMed]
- Takeda, S.I.; Maeda, H.; Hayakawa, M.; Sawabata, N.; Maekura, R. Current surgical intervention for pulmonary tuberculosis. Ann. Thorac. Surg. 2005, 79, 959–963. [Google Scholar]
- Olcmen, A.; Gunluoglu, M.Z.; Demir, A.; Akin, H.; Kara, H.V.; Dincer, S.I. Role and outcome of surgery for pulmonary tuberculosis. Asian Cardiovasc. Thorac. Ann. 2006, 14, 363–366. [Google Scholar]
- Perelman, M.I.; Strelzov, V.P. Surgery for pulmonary tuberculosis. World J. Surg. 1997, 21, 457–467. [Google Scholar]
- Andrews, J.R.; Gandhi, N.R.; Moodley, P.; Shah, N.S.; Bohlken, L.; Moll, A.P.; Pillay, M.; Friedland, G.; Sturm, A.W. Tugela Ferry Care and Research Collaboration. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J. Infect. Dis. 2008, 198, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Shakow, A.; Mate, K.; Vanderwarker, C.; Gupta, R.; Farmer, P. Limited good and limited vision: multidrug-resistant tuberculosis and global health policy. Soc. Sci. Med. 2005, 61, 847–859. [Google Scholar]
- Espinal, M.A.; Kim, S.J.; Suarez, P.G.; Kam, K.M.; Khomenko, A.G.; Migliori, G.B.; Baéz, J.; Kochi, A.; Dye, C.; Raviglione, M.C. Standard short-course chemotherapy for drug-resistant tuberculosis. JAMA 2000, 283, 2537–2545. [Google Scholar]
- Furin, J.J.; Becerra, M.C.; Shin, S.S.; Kim, J.Y.; Bayona, J.; Farmer, P.E. Effect of administering short-course standardized, regimens in individuals infected with drug-resistant Mycobacterium tuberculosis strains. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Reichman, L. Timebomb: the Global Epidemic of Multi-Drug Resistant Tuberculosis; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis. 1999, 5, 18–27. [Google Scholar]
- Tang, S.; Squire, S.B. What lessons can de drawn from tuberculosis (TB) control in China in the 1990s? An analysis from a health system perspective. Health Policy 2005, 72, 93–104. [Google Scholar]
- World Health Organization. Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Weekly Epidemiol. Rec. 2006, 81, 430–432.
- Chan, E.D.; Strand, M.J.; Iseman, M.D. Treatment outcomes in extensively resistant tuberculosis. New Engl. J. Med. 2008, 359, 657–659. [Google Scholar]
- Hopewell, P.C.; Pai, M.; Maher, D.; Uplekar, M.; Raviglione, M.C. International standards for tuberculosis care. Lancet Infect. Dis. 2006, 6, 710–725. [Google Scholar]
- Zignol, M.; Hosseini, M.S.; Wright, A.; Lambregts-van Weezenbeek, C.; Nunn, P.; Watt, C.J.; Williams, B.G.; Dye, C. Global incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 2006, 194, 479–485. [Google Scholar]
- Dye, C.; Williams, B.G. Criteria for the control of drug-resistant tuberculosis. Proc. Natl. Acad. Sci. USA 2000, 97, 8180–8185. [Google Scholar]
- World Health Organization, Anti-Tuberculosis Drug Resistance in the World. Report No. 4; World Health Organization: Geneva, Switzerland, 2008.
- Keshavjee, S.; Gelmanova, I.Y.; Farmer, P.E.; Mishustin, S.P.; Strelis, A.K.; Andreev, Y.G.; Pasechnikov, A.D.; Atwood, S.; Mukherjee, J.S.; Rich, M.L.; et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet 2008, 372, 1403–1409. [Google Scholar] [PubMed]
- Kim, D.H.; Kim, H.J.; Park, S.K.; Kong, S.J.; Kim, Y.S.; Kim, T.H.; Kim, E.K.; Lee, K.M.; Lee, S.S.; Park, J.S.; Koh, W.J.; Lee, C.H.; Kim, J.Y.; Shim, T.S. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 2008, 178, 1075–1082. [Google Scholar]
- Nachega, J.B.; Chaisson, R.E. Tuberculosis drug resistance: a global threat. Clin. Infect. Dis. 2003, 36, S24–30. [Google Scholar]
- Zhang, Y.; Heym, B.; Allen, B.; Young, D.; Cole, S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 1992, 358, 591–593. [Google Scholar] [PubMed]
- Banerjee, A.; Dubnau, E.; Quemard, A.; Balasubramanian, V.; Um, K.S.; Wilson, T.; Collins, D.; de Lisle, G.; Jacobs, W.R., Jr. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994, 263, 227–230. [Google Scholar] [PubMed]
- Miesel, L.; Weisbrod, T.R.; Marcinkeviciene, J.A.; Bittman, R.; Jacobs, W.R., Jr. NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J. Bacteriol. 1998, 180, 2459–2467. [Google Scholar] [PubMed]
- Lee, A.S.G.; Teo, A.S.M.; Wong, S.Y. Novel mutations in ndh in isoniazid-resistant Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2001, 45, 2157–2159. [Google Scholar] [PubMed]
- Telenti, A.; Imboden, P.; Marchesi, F.; Lowrie, D.; Cole, S.; Colston, M.J.; Matter, L.; Schopfer, K.; Bodmer, T. Detection of rifampin-resistant mutations in Mycobacterium tuberculosis. Lancet 1993, 341, 647–650. [Google Scholar] [PubMed]
- Miller, L.P.; Crawford, J.T.; Shinnick, T.M. The rpoB gene of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1994, 38, 805–811. [Google Scholar] [PubMed]
- Williams, D.L.; Spring, L.; Collins, L.; Miller, L.P.; Heifets, L.B.; Gangadharam, P.R.; Gillia, T.P. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1998, 42, 1853–1857. [Google Scholar] [PubMed]
- Scorpio, A.; Lindholm-Levy, P.; Heifets, L.; Gilman, R.; Siddiqi, S.; Cynamon, M.; Zhang, Y. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1997, 41, 540–543. [Google Scholar] [PubMed]
- Konno, K.; Feldmann, F.M.; McDermott, W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am. Rev. Respir. Dis. 1967, 95, 461–469. [Google Scholar]
- Sreevatsan, S.; Stockbauer, K.E.; Pan, X.; Kreiswirth, B.N.; Moghazeh, S.L.; Jacobs, W.R., Jr.; Telenti, A.; Musser, J.M. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob. Agents Chemother. 1997, 41, 1677–1681. [Google Scholar] [PubMed]
- Telenti, A.; Philipp, W.J.; Sreevatsan, S.; Bernasconi, C.; Stockbauer, K.E.; Wieles, B.; Musser, J.M.; Jacobs, W.R., Jr. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 1997, 3, 567–570. [Google Scholar] [PubMed]
- Ramaswamy, S.V.; Amin, A.G.; Goksel, S.; Stager, C.E.; Dou, S.J.; El Sahly, H.; Moghazeh, S.L.; Kreiswirth, B.N.; Musser, J.M. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2000, 44, 326–336. [Google Scholar] [PubMed]
- Finken, M.; Kirschner, P.; Meier, A.; Wrede, A.; Böttger, E.C. Molecular basis of streptomycin resistance in Mycobacterium tuberculosis: alterations of the ribosomal protein S12 gene and point mutations within a functional 16S ribosomal RNA pseudoknot. Mol. Microbiol. 1993, 9, 1239–1246. [Google Scholar] [PubMed]
- Nair, J.; Rouse, D.A.; Bai, G.H.; Morris, S.L. The rpsL gene and streptomycin resistance in single and multiple drug-resistant strains of Mycobacterium tuberculosis. Mol. Microbiol. 1993, 10, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Kirschner, P.; Bange, F.C.; Vogel, U.; Böttger, E.C. Genetic alterations in streptomycin-resistant Mycobacterium tuberculosis: mapping of mutations conferring resistance. Antimicrob. Agents Chemother. 1994, 38, 228–233. [Google Scholar] [PubMed]
- Honore, N.; Cole, S.T. Streptomycin resistance in mycobacteria. Antimicrob. Agents Chemother. 1994, 38, 238–242. [Google Scholar]
- Alangaden, G.J.; Kreiswirth, B.N.; Aouad, A.; Khetarpal, M.; Igno, F.R.; Moghazeh, S.L.; Manavathu, E.K.; Lerner, S.A. Mechanism of resistance to amikacin and kanamycin in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1998, 42, 1295–1297. [Google Scholar] [PubMed]
- Suzuki, Y.; Katsukawa, C.; Tamaru, A.; Abe, C.; Makino, M.; Mizuguchi, Y.; Taniguchi, H. Detection of kanamycin-resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. J. Clin. Microbiol. 1998, 36, 1220–1225. [Google Scholar] [PubMed]
- Taniguchi, H.; Chang, B.; Abe, C.; Nikaido, Y.; Mizuguchi, Y.; Yoshida, S.I. Molecular analysis of kanamycin and viomycin resistance in Mycobacterium smegmatis by use of the conjugation system. J. Bacteriol. 1997, 179, 4795–4801. [Google Scholar] [PubMed]
- McClatchy, J.K.; Kanes, W.; Davidson, P.T.; Moulding, T.S. Cross-resistance in M. tuberculosis to kanamycin, capreomycin and viomycin. Tubercle 1977, 58, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tsukamura, M.; Mizuno, S. Cross-resistant relationships among the aminoglucoside antibiotics in Mycobacterium tuberculosis. J. Gen. Microbiol. 1975, 88, 269–274. [Google Scholar] [PubMed]
- Maus, C.E.; Plikaytis, B.B.; Shinnick, T.M. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculos. Antimicrob. Agents Chemother. 2005, 49, 3192–3197. [Google Scholar] [PubMed]
- Maus, C.E.; Plikaytis, B.B.; Shinnick, T.M. Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2005, 49, 571–577. [Google Scholar] [PubMed]
- Takiff, H.E.; Salazar, L.; Guerrero, C.; Philipp, W.; Huang, W.M.; Kreiswirth, B.; Cole, S.T.; Jacobs, W.R., Jr.; Telenti, A. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 1994, 38, 773–780. [Google Scholar] [PubMed]
- Cambau, E.; Sougakoff, W.; Besson, M.; Truffot-Pernot, C.; Grosset, J.; Jarlier, V. Selection of a gyrA mutant of Mycobacterium tuberculosis resistant to fluoroquinolones during treatment with ofloxacin. J. Infect. Dis. 1994, 170, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.P.; Metchock, B.; Sikes, D.; Crawford, J.T.; Cooksey, R.C. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2003, 47, 3799–3805. [Google Scholar] [PubMed]
- DeBarber, A.E.; Mdluli, K.; Bosman, M.; Bekker, L.G.; Barry, C.E., 3rd. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9677–9682. [Google Scholar]
- Baulard, A.R.; Betts, J.C.; Engohang-Ndong, J.; Quan, S.; McAdam, R.A.; Brennan, P.J.; Locht, C.; Besra, G.S. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 2000, 275, 28326–28331. [Google Scholar]
- Rengarajan, J.; Sassetti, C.M.; Naroditskaya, V.; Sloutsky, A.; Bloom, B.R.; Rubin, E.J. The folate pathway is a target for resistance to the drug para-aminosalicylic acid (PAS) in mycobacteria. Mol. Microbiol. 2004, 53, 275–282. [Google Scholar]
- Mathys, V.; Wintjens, R.; Lefevre, P.; Bertout, J.; Singhal, A.; Kiass, M.; Kurepina, N.; Wang, X.M.; Mathema, B.; Baulard, A.; Kreiswirth, B.N.; Bifani, P. Molecular genetics of para-aminosalicylic acid resistance in clinical isolates and spontaneous mutants of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2009, 53, 2100–2109. [Google Scholar] [PubMed]
- Chacon, O.; Feng, Z.; Harris, N.B.; Caceres, N.E.; Adams, L.G.; Barletta, R.G. Mycobacterium smegmatis D-alanine racemase mutants are not dependent on D-alanine for growth. Antimicrob. Agents Chemother. 2002, 46, 47–54. [Google Scholar] [PubMed]
- Caceres, N.E.; Harris, N.B.; Wellehan, J.F.; Feng, Z.; Kapur, V.; Barletta, R.G. Overexpression of the D-alanine racemase gene confers resistance to D-cycloserine in Mycobacterium smegmatis. J. Bacteriol. 1997, 179, 5046–5055. [Google Scholar] [PubMed]
- Das, S.; Yennamalli, R.M.; Vishnoi, A.; Gupta, P.; Bhattacharya, A. Single-nucleotide variations associated with Mycobacterium tuberculosis KwaZulu-Natal strains. J. Biosci. 2009, 34, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, A.S.; Dai, Y.; Jones-López, E.C.; Hwang, S.H.; Lee, J.S.; Cho, S.N.; Via, L.E.; Barry, C.E., III.; Alland, D. Mutations in extensively drug-resistant Mycobacterium tuberculosis that do not code for known drug-resistance mechanisms. J. Infect. Dis. 2010, 201, 881–888. [Google Scholar] [PubMed]
- Nathanson, E.; Weezenbeek, C.L.V.; Ric, M.L.; Gupta, R.; Bayona, J.; Blöndal, K.; Caminero, J.A.; Cegielski, J.P.; Danilovits, M.; Espinal, M.A.; et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg. Infect. Dis. 2006, 12, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Raviglione, M.C. XDR-TB: entering the post-antibiotic era? Int. J. Tuberc. Lung Dis. 2006, 10, 1185–1187. [Google Scholar] [PubMed]
- Matteelli, A.; Migliori, G.B.; Cirillo, D.; Centis, R.; Girardi, E.; Raviglione, M. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Rev. Anti. Infect. Ther. 2007, 5, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; Besozzi, G.; Girardi, E.; Kliiman, K.; Lange, C.; Toungoussovae, O.S.; Ferrara, G.; Cirillo, D.M.; Gori, A.; Matteelli, A.; Spanevelloe, A.; Codecasa, L.R.; Raviglione, M.C. SMIRA/TBNET Study Group. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur. Respir. J. 2007, 30, 623–626. [Google Scholar] [PubMed]
- Orenstein, E.W.; Basu, S.; Shah, N.S.; Andrews, J.R.; Friedland, G.H.; Moll, A.P.; Gandhi, N.R.; Galvani, A.P. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 153–161. [Google Scholar]
- Orenstein, E.W.; Galvani, A.P. Treatment outcomes among patients with multidrug-resistant tuberculosis-Authors' reply. Lancet Infect. Dis. 2010, 10, 215–216. [Google Scholar]
- Gandhi, N.R.; Moll, A.; Sturm, A.W.; Pawinski, R.; Govender, T.; Lalloo, U.; Zeller, K.; Andrews, J.; Friedland, G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural areas of South Africa. Lancet 2006, 368, 1575–1580. [Google Scholar]
- Koenig, R. In South Africa, XDR TB and HIV prove a deadly combination. Science 2008, 319, 894–897. [Google Scholar]
- Loveday, M.; Thomson, L.; Chopra, M.; Ndlela, Z. A health systems assessment of the KwaZulu-Natal tuberculosis programme in the context of increasing drug resistance. Int. J. Tuberc. Lung Dis. 2008, 12, 1042–1047. [Google Scholar]
- Shah, N.S.; Wright, A.; Bai, G.H.; Barrera, L.; Boulahbal, F.; Martín-Casabona, N.; Drobniewski, F.; Gilpin, C.; Havelková, M.; Lepe, R.; et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 2007, 13, 380–387. [Google Scholar] [PubMed]
- Masjedi, M.R.; Farnia, P.; Sorooch, S.; Pooramiri, M.V.; Mansoori, S.D.; Zarifi, A.Z.; Akbarvelayati, A.; Hoffner, S. Extensively drug-resistant tuberculosis: 2 years of surveillance in Iran. Clin. Infect. Dis. 2006, 43, 841–847. [Google Scholar]
- Migliori, G.B.; Ortmann, J.; Girardi, E.; Besozzi, G.; Lange, C.; Cirillo, D.M.; Ferrarese, M.; Iaco, G.D.; Gori, A.; Raviglione, M.C.; Group, S.T.S. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg. Infect. Dis. 2007, 13, 780–782. [Google Scholar] [PubMed]
- Kim, H.R.; Hwang, S.S.; Kim, H.J.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; Yim, J.J. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin. Infect. Dis. 2007, 45, 1290–1295. [Google Scholar]
- Mitnick, C.D.; Shin, S.S.; Seung, K.J.; Rich, M.L.; Atwood, S.S.; Furin, J.J.; Fitzmaurice, G.M.; Viru, F.A.A.; Appleton, S.C.; Bayona, J.N.; et al. Comprehensive treatment of extensively drug-resistant tuberculosis. New Engl. J. Med. 2008, 359, 563–574. [Google Scholar]
- Hamilton, C.D.; Sterling, T.R.; Blumberg, H.M.; Leonard, M.; McAuley, J.; Schlossberg, D.; Stout, J.; Huitt, G. Extensively drug-resistant tuberculosis: are we learning from history or repeating it? Clin. Infect. Dis. 2007, 45, 338–342. [Google Scholar] [PubMed]
- Jakubowiak, W.M.; Bogorodskaya, E.M.; Borisov, S.E.; Danilova, I.D.; Lomakina, O.B.; Kourbatova, E.V. Impact of socio-psychological factors on treatment adherence of TB patients in Russia. Tuberculosis 2008, 88, 495–502. [Google Scholar]
- White, V.L.C.; Moore-Gillon, J. Resource implications of patients with multidrug resistant tuberculosis. Thorax 2000, 55, 962–963. [Google Scholar]
- Rajbhandary, S.S.; Marks, S.M.; Bock, N.N. Costs of patients hospitalized for multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2004, 8, 1012–1016. [Google Scholar]
- Iseman, M.D. Treatment of multidrug-resistant tuberculosis. New Engl. J. Med. 1993, 329, 784–791. [Google Scholar]
- Mdluli, K.; Spigelman, M. Novel targets for tuberculosis drug discovery. Curr. Opin. Pharmacol. 2006, 6, 459–467. [Google Scholar]
- Spigelman, M.; Gillespie, S. Tuberculosis drug development pipeline: progress and hope. Lancet 2006, 367, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 529–564. [Google Scholar]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; Williams, P.; de Chaffoy, D.; Huitric, E.; Hoffner, S.; Cambau, E.; Truffot-Pernot, C.; Lounis, N.; Jarlier, V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 223–227. [Google Scholar]
- Koul, A.; Vranckx, L.; Dendouga, N.; Balemans, W.; Van den Wyngaert, I.; Vergauwen, K.; Göhlmann, H.W.H.; Willebrords, R.; Poncelet, A.; Guillemont, J.; Bald, D.; Andries, K. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J. Biol. Chem. 2008, 283, 25273–25280. [Google Scholar]
- Diacon, A.H.; Pym, A.; Grobusch, M.; Patientia, R.; Rustomjee, R.; Page-Shipp, L.; Pistorius, C.; Krause, R.; Bogoshi, M.; Churchyard, G.; et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. New Engl. J. Med. 2009, 360, 2397–2405. [Google Scholar]
- Rustomjee, R.; Diacon, A.H.; Allen, J.; Venter, A.; Reddy, C.; Patientia, R.F.; Mthiyane, T.C.P.; Marez, T.D.; van Heeswijk, R.; Kerstens, R.; Koul, A.; Beule, K.D.; Donald, P.R.; McNeeley, D.F. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 2831–2835. [Google Scholar]
- Stover, C.K.; Warrener, P.; Van Devanter, D.R.; Sherman, D.R.; Arain, T.M.; Langhorne, M.H.; Anderson, S.W.; Towell, J.A.; Yuan, Y.; McMurray, D.N.; et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 2000, 405, 962–966. [Google Scholar] [PubMed]
- Singh, R.; Manjunatha, U.; Boshoff, H.I.M.; Ha, Y.H.; Niyomrattanakit, P.; Ledwidge, R.; Dowd, C.S.; Lee, I.Y.; Kim, P.; Zhang, L.; et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 2008, 322, 1392–1395. [Google Scholar] [PubMed]
- Manjunatha, U.; Boshoff, H.I.; Barry, C.E., III. The mechanism of action of PA-824: novel insights from transcriptional profiling. Commun. Integr. Biol. 2009, 2, 215–218. [Google Scholar]
- Kremer, L.; Besra, G.S. A waxy tale, by Mycobacterium tuberculosis. In Tuberculosis and the tubercle bacillus; Cole, S.T., Eisenach, K.D., McMurray, D.N., Jacobs, W.R., Jr., Eds.; ASM Press: Washington, D.C., USA, 2005; pp. 287–305. [Google Scholar]
- Lenaerts, A.J.; Gruppo, V.; Marietta, K.S.; Johnson, C.M.; Driscoll, D.K.; Tompkins, N.M.; Rose, J.D.; Reynolds, R.C.; Orme, I.M. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 2005, 49, 2294–2301. [Google Scholar] [PubMed]
- Tyagi, S.; Nuermberger, E.; Yoshimatsu, T.; Williams, K.; Rosenthal, I.; Lounis, N.; Bishai, W.; Grosset, J. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2005, 49, 2289–2293. [Google Scholar]
- Nuermberger, E.; Tyagi, S.; Tasneen, R.; Williams, K.N.; Almeida, D.; Rosenthal, I.; Grosset, J.H. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 1522–1524. [Google Scholar] [PubMed]
- Ginsberg, A.M.; Laurenzi, M.W.; Rouse, D.J.; Whitney, K.D.; Spigelman, M.K. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 2009, 53, 3720–3725. [Google Scholar] [PubMed]
- Ginsberg, A.M.; Laurenzi, M.W.; Rouse, D.J.; Whitney, K.D.; Spigelman, M.K. Assessment of the effects of the nitroimidazo-oxazine, PA-824, on renal function in healthy subjects. Antimicrob. Agents Chemother. 2009, 53, 3726–3733. [Google Scholar] [PubMed]
- Matsumoto, M.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Tsubouchi, H.; Sasaki, H.; Shimokawa, Y.; Komatsu, M. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006, 3, 2131–2144. [Google Scholar]
- Saliu, O.Y.; Crismale, C.; Schwander, S.K.; Wallis, R.S. Bactericidal activity of OPC-67683 against drug-tolerant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2007, 60, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Haraguchi, Y.; Itotani, M.; Kuroda, H.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Matsumoto, M.; Komatsu, M.; Tsubouchi, H. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles. J. Med. Chem. 2006, 49, 7854–7860. [Google Scholar] [PubMed]
- Wayne, L.G.; Sramek, H.A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1994, 38, 2054–2058. [Google Scholar] [PubMed]
- Showalter, H.D.H.; Denny, W.A. A roadmap for drug discovery and its translation to small molecule agents in clinical development for tuberculosis treatment. Tuberculosis 2008, 88, S3–S17. [Google Scholar]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar]
- World Health Organization, Antimicrobial Resistance, Fact Sheet No. 194; World Health Organization: Geneva, Switzerland, 2002.
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Haydel, S.E. Extensively Drug-Resistant Tuberculosis: A Sign of the Times and an Impetus for Antimicrobial Discovery. Pharmaceuticals 2010, 3, 2268-2290. https://0-doi-org.brum.beds.ac.uk/10.3390/ph3072268
Haydel SE. Extensively Drug-Resistant Tuberculosis: A Sign of the Times and an Impetus for Antimicrobial Discovery. Pharmaceuticals. 2010; 3(7):2268-2290. https://0-doi-org.brum.beds.ac.uk/10.3390/ph3072268
Chicago/Turabian StyleHaydel, Shelley E. 2010. "Extensively Drug-Resistant Tuberculosis: A Sign of the Times and an Impetus for Antimicrobial Discovery" Pharmaceuticals 3, no. 7: 2268-2290. https://0-doi-org.brum.beds.ac.uk/10.3390/ph3072268




