Mouse and Fly Sperm Motility Changes Differently under Modelling Microgravity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Estimation of the Sperm Motility
2.3. Western Blotting
2.4. Statistical Analysis
3. Results
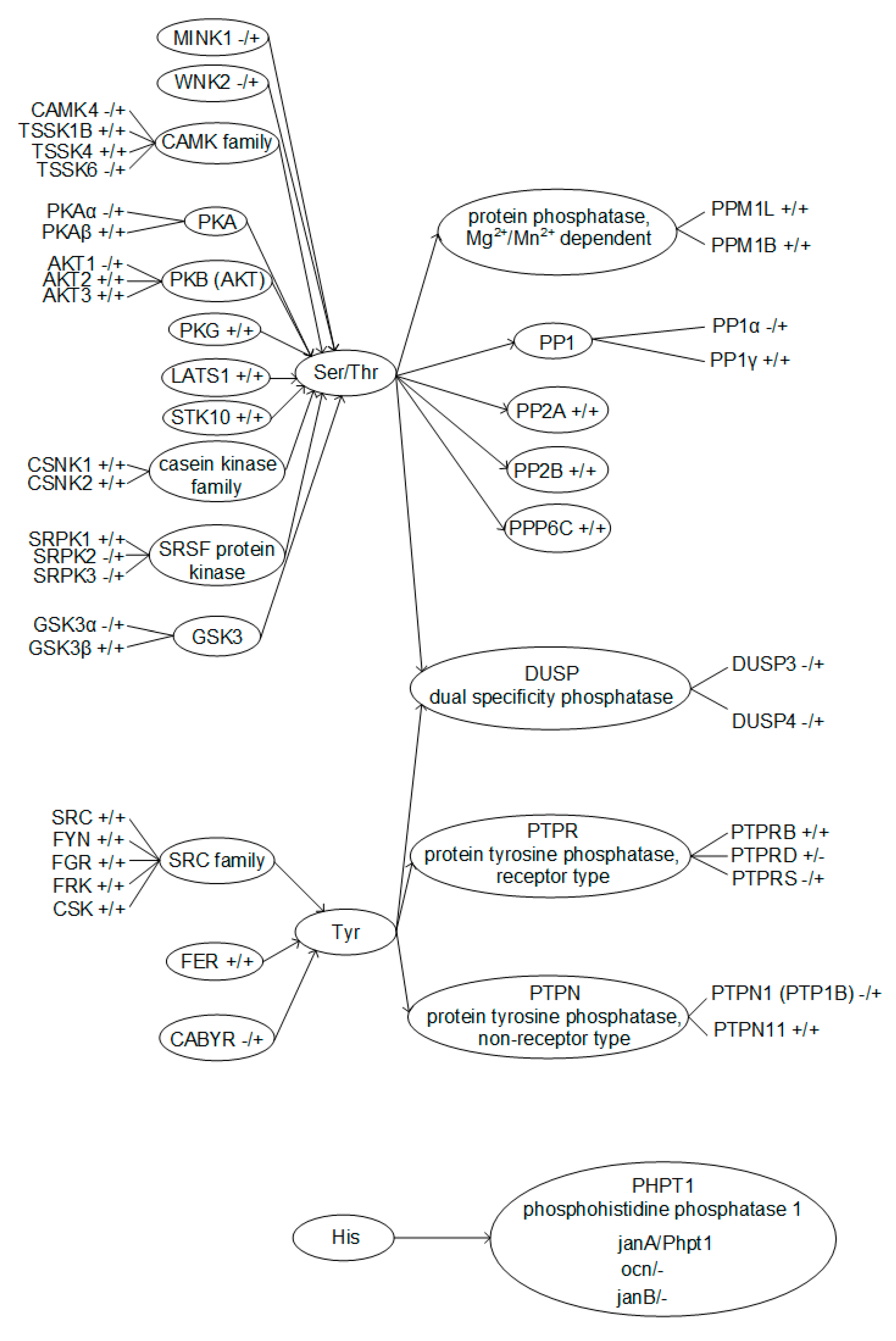
3.1. Database Search
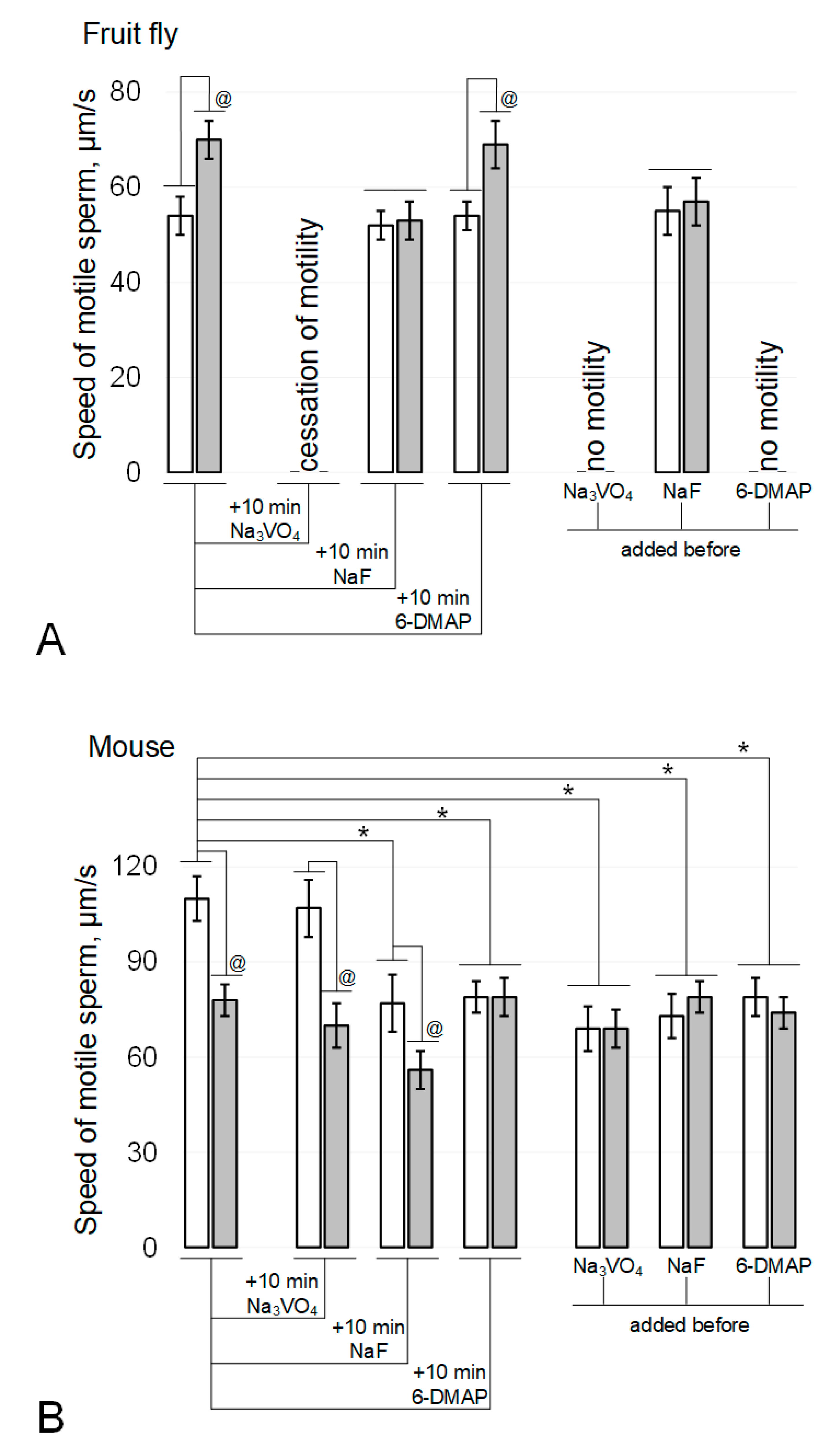
3.2. Sperm Motility
3.3. Protein Content
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inaba, K. Molecular Architecture of the Sperm Flagella: Molecules for Motility and Signaling. Zoöl. Sci. 2003, 20, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, I.R. Dynein family of motor proteins: Present status and future questions. Cell Motil. Cytoskelet. 1995, 32, 136–144. [Google Scholar] [CrossRef]
- Inaba, K. Sperm flagella: Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 2011, 17, 524–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, M.E.; Sale, W.S. The 9+2 Axoneme Anchors Multiple Inner Arm Dyneins and a Network of Kinases and Phosphatases That Control Motility. J. Cell Biol. 2000, 151, F37–F42. [Google Scholar] [CrossRef] [PubMed]
- Wirschell, M.; Yamamoto, R.; Alford, L.; Gokhale, A.; Gaillard, A.; Sale, W.S. Regulation of ciliary motility: Conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch. Biochem. Biophys. 2011, 510, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Tash, J.S.; Bracho, G.E. Microgravity alters protein phosphorylation changes during initiation of sea urchin sperm motility. FASEB J. 1999, 13, S43–S54. [Google Scholar] [CrossRef]
- Tash, J.S.; Kim, S.; Schuber, M.; Seibt, D.; Kinsey, W.H. Fertilization of Sea Urchin Eggs and Sperm Motility Are Negatively Impacted under Low Hypergravitational Forces Significant to Space Flight1. Biol. Reprod. 2001, 65, 1224–1231. [Google Scholar] [CrossRef] [Green Version]
- Ogneva, I.V.; Usik, M.A.; Burtseva, M.V.; Biryukov, N.S.; Zhdankina, Y.S.; Sychev, V.N.; Orlov, O.I. Drosophila melanogaster Sperm under Simulated Microgravity and a Hypomagnetic Field: Motility and Cell Respiration. Int. J. Mol. Sci. 2020, 21, 5985. [Google Scholar] [CrossRef]
- Kamiya, H.; Sasaki, S.; Ikeuchi, T.; Umemoto, Y.; Tatsura, H.; Hayashi, Y.; Kaneko, S.; Kohri, K. Effect of Simulated Microgravity on Testosterone and Sperm Motility in Mice. J. Androl. 2003, 24, 885–890. [Google Scholar] [CrossRef]
- Usik, M.A.; Ogneva, I.V. Cytoskeleton Structure in Mouse Sperm and Testes After 30 Days of Hindlimb Unloading and 12 Hours of Recovery. Cell. Physiol. Biochem. 2018, 51, 375–392. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Usik, M.A.; Biryukov, N.S.; Zhdankina, Y.S. Sperm Motility of Mice under Simulated Microgravity and Hypergravity. Int. J. Mol. Sci. 2020, 21, 5054. [Google Scholar] [CrossRef]
- Ikeuchi, T.; Sasaki, S.; Umemoto, Y.; Kubota, Y.; Kubota, H.; Kaneko, T.; Kohri, K. Human sperm motility in a microgravity environment. Reprod. Med. Biol. 2005, 4, 161–168. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.J. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Towbin, H.; Staehlin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Proce-dure and some application. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorus, S.; Busby, S.A.; Gerike, U.; Shabanowitz, J.; Hunt, N.F.; Karr, T.L. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat. Genet. 2006, 38, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Karr, T.L. Fruit flies and the sperm proteome. Hum. Mol. Genet. 2007, 16, R124–R133. [Google Scholar] [CrossRef]
- Baker, M.A.; Hetherington, L.; Reeves, G.M.; Aitken, R.J. The mouse sperm proteome characterizedvia IPG strip prefractionation and LC-MS/MS identification. Proteom. 2008, 8, 1720–1730. [Google Scholar] [CrossRef]
- Cao, W.; Gerton, G.; Moss, S.B. Proteomic Profiling of Accessory Structures from the Mouse Sperm Flagellum. Mol. Cell. Proteom. 2006, 5, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, R.; Goswami, S.; Dudiki, T.; Popkie, A.P.; Phiel, C.J.; Kline, U.; Vijayaraghavan, S. Targeted Disruption of Glycogen Synthase Kinase 3a (Gsk3a) in Mice Affects Sperm Motility Resulting in Male Infertility1. Biol. Reprod. 2015, 92, 65. [Google Scholar] [CrossRef]
- Dey, S.; Goswami, S.; Eisa, A.; Bhattacharjee, R.; Brothag, C.; Kline, D.; Vijayaraghavan, S. Cyclic AMP and glycogen synthase kinase 3 form a regulatory loop in spermatozoa. J. Cell. Physiol. 2018, 233, 7239–7252. [Google Scholar] [CrossRef]
- Tash, J.S.; Bracho, G.E. Identification of Phosphoproteins Coupled to Initiation of Motility in Live Epididymal Mouse Sperm. Biochem. Biophys. Res. Commun. 1998, 251, 557–563. [Google Scholar] [CrossRef]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals. Front. Cell Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Byrum, C.; Walton, K.; Robertson, A.; Carbonneau, S.; Thomason, R.; Coffman, J.; McClay, D. Protein tyrosine and serine–threonine phosphatases in the sea urchin, Strongylocentrotus purpuratus: Identification and potential functions. Dev. Biol. 2006, 300, 194–218. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.G.; Ortega-Ferrusola, C.; Garcia, B.M.; Salido, G.M.; Peña, F.J.; Tapia, J.A. Identification of Protein Tyrosine Phosphatases and Dual-Specificity Phosphatases in Mammalian Spermatozoa and Their Role in Sperm Motility and Protein Tyrosine Phosphorylation1. Biol. Reprod. 2009, 80, 1239–1252. [Google Scholar] [CrossRef]
- Baker, M.A.; Hetherington, L.; Aitken, R.J. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J. Cell Sci. 2006, 119, 3182–3192. [Google Scholar] [CrossRef] [Green Version]
- Varano, G.; Lombardi, A.; Cantini, G.; Forti, G.; Baldi, E.; Luconi, M. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum. Reprod. 2008, 23, 2652–2662. [Google Scholar] [CrossRef] [Green Version]
- Krapf, D.; Arcelay, E.; Wertheimer, E.V.; Sanjay, A.; Pilder, S.H.; Salicioni, A.M.; Visconti, P.E. Inhibition of Ser/Thr Phosphatases Induces Capacitation-associated Signaling in the Presence of Src Kinase Inhibitors. J. Biol. Chem. 2010, 285, 7977–7985. [Google Scholar] [CrossRef] [Green Version]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; De la Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef] [Green Version]
- Tomes, C.; Roggero, C.; De Blas, G.; Saling, P.; Mayorga, L. Requirement of protein tyrosine kinase and phosphatase activities for human sperm exocytosis. Dev. Biol. 2004, 265, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Yanicostas, C.; Vincent, A.; A Lepesant, J. Transcriptional and posttranscriptional regulation contributes to the sex-regulated expression of two sequence-related genes at the janus locus of Drosophila melanogaster. Mol. Cell. Biol. 1989, 9, 2526–2535. [Google Scholar] [CrossRef] [Green Version]
- Hardman, G.; Perkins, S.; Brownridge, P.J.; Clarke, C.J.; Byrne, D.P.; Campbell, A.; Kalyuzhnyy, A.; Myall, A.; Eyers, P.A.; Jones, A.; et al. Strong anion exchange-mediated phosphoproteomics reveals extensive human non-canonical phosphorylation. EMBO J. 2019, 38, e100847. [Google Scholar] [CrossRef]
- Ma, H. Requirement of the Inositol Trisphosphate Receptor for Activation of Store-Operated Ca2+ Channels. Science 2000, 287, 1647–1651. [Google Scholar] [CrossRef]
- Patterson, R.L.; van Rossum, D.; Gill, D.L. Store-Operated Ca2+ Entry: Evidence for a Secretion-like Coupling Model. Cell 1999, 98, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Downey, G.P.; Takai, A.; Zamel, R.; Grinstein, S.; Chan, C.K. Okadaic acid-induced actin assembly in neutrophils: Role of protein phosphatases. J. Cell. Physiol. 1993, 155, 505–519. [Google Scholar] [CrossRef]
- Kreienbuhl, P.; Keller, H.; Niggli, V. Protein phosphatase inhibitors okadaic acid and calyculin A alter cell shape and F-actin distribution and inhibit stimulus-dependent increases in cytoskeletal actin of human neutrophils. Blood 1992, 80, 2911–2919. [Google Scholar] [CrossRef]
- Hosoya, N.; Mitsui, M.; Yazama, F.; Ishihara, H.; Ozaki, H.; Karaki, H.; Hartshorne, D.J.; Mohri, H. Changes in the cytoskeletal struc-ture of cultured smooth muscle cells induced by calyculin-A. J. Cell Sci. 1993, 105, 883–890. [Google Scholar] [CrossRef]
- Shurety, W.; Stewart, N.L.; Stow, J. Fluid-Phase Markers in the Basolateral Endocytic Pathway Accumulate in Response to the Actin Assembly-promoting Drug Jasplakinolide. Mol. Biol. Cell 1998, 9, 957–975. [Google Scholar] [CrossRef] [Green Version]
- Ogneva, I.V.; Biryukov, N.S. Lecithin Prevents Cortical Cytoskeleton Reorganization in Rat Soleus Muscle Fibers under Short-Term Gravitational Disuse. PLoS ONE 2016, 11, e0153650. [Google Scholar] [CrossRef] [Green Version]
- Ogneva, I.V. Cell Mechanosensitivity: Mechanical Properties and Interaction with Gravitational Field. BioMed Res. Int. 2012, 2013, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ogneva, I.V.; Biryukov, N.S.; Leinsoo, T.A.; Larina, I.M. Possible Role of Non-Muscle Alpha-Actinins in Muscle Cell Mechanosensitivity. PLoS ONE 2014, 9, e96395. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Maximova, M.V.; Larina, I.M. Structure of cortical cytoskeleton in fibers of mouse muscle cells after being exposed to a 30-day space flight on board the BION-M1 biosatellite. J. Appl. Physiol. 2014, 116, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Ogneva, I.V.; Gnyubkin, V.; Laroche, N.; Maximova, M.V.; Larina, I.M.; Vico, L. Structure of the cortical cytoskeleton in fibers of postural muscles and cardiomyocytes of mice after 30-day 2-g centrifugation. J. Appl. Physiol. 2015, 118, 613–623. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogneva, I.V. Mouse and Fly Sperm Motility Changes Differently under Modelling Microgravity. Curr. Issues Mol. Biol. 2021, 43, 590-604. https://0-doi-org.brum.beds.ac.uk/10.3390/cimb43020043
Ogneva IV. Mouse and Fly Sperm Motility Changes Differently under Modelling Microgravity. Current Issues in Molecular Biology. 2021; 43(2):590-604. https://0-doi-org.brum.beds.ac.uk/10.3390/cimb43020043
Chicago/Turabian StyleOgneva, Irina V. 2021. "Mouse and Fly Sperm Motility Changes Differently under Modelling Microgravity" Current Issues in Molecular Biology 43, no. 2: 590-604. https://0-doi-org.brum.beds.ac.uk/10.3390/cimb43020043





