Curcumin Effect on Copper Transport in HepG2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culturing, Treatment and Sample Collection
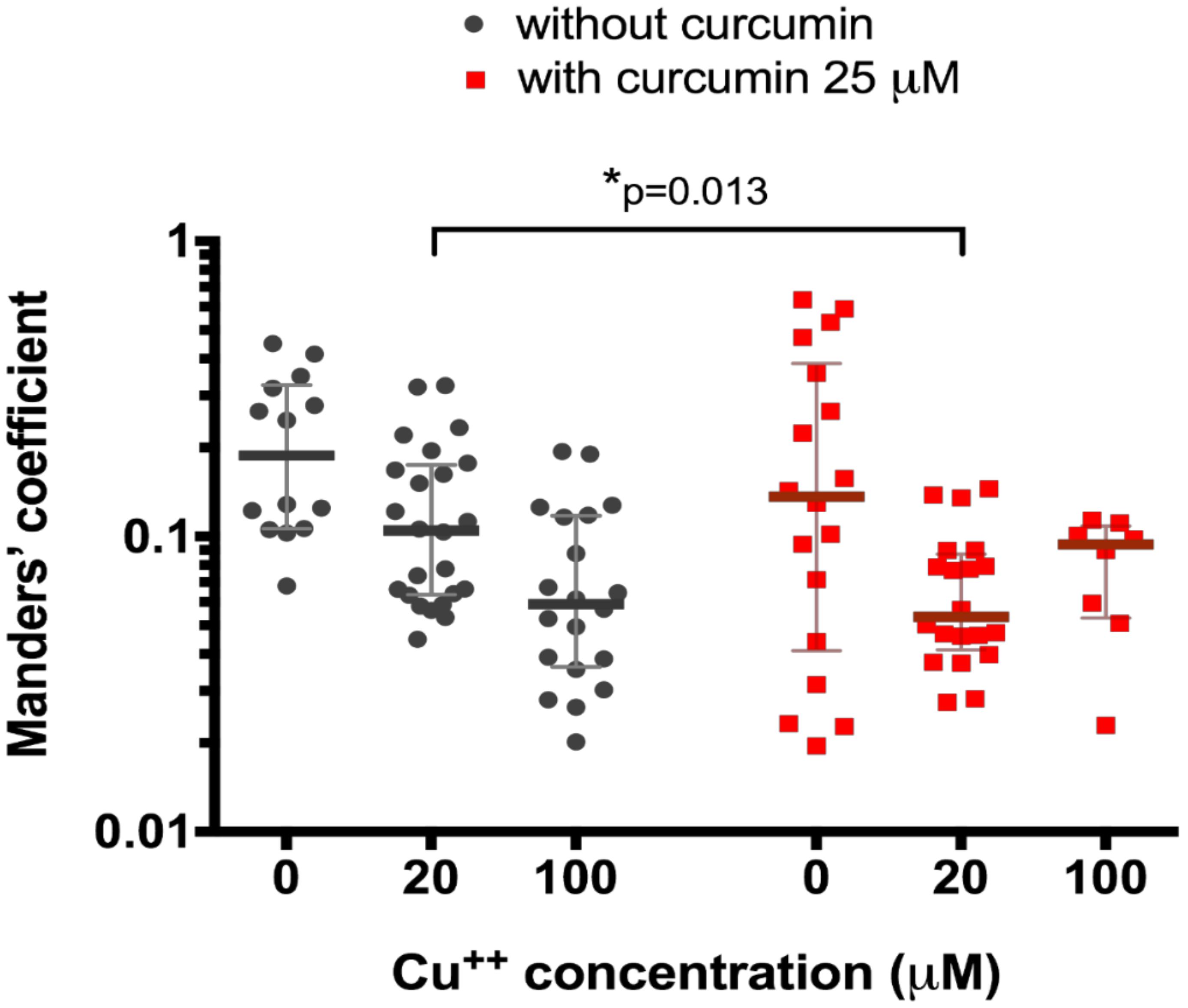
2.2. Immunofluorescent Stainings, Confocal Microscopy and Co-Localization Analysis
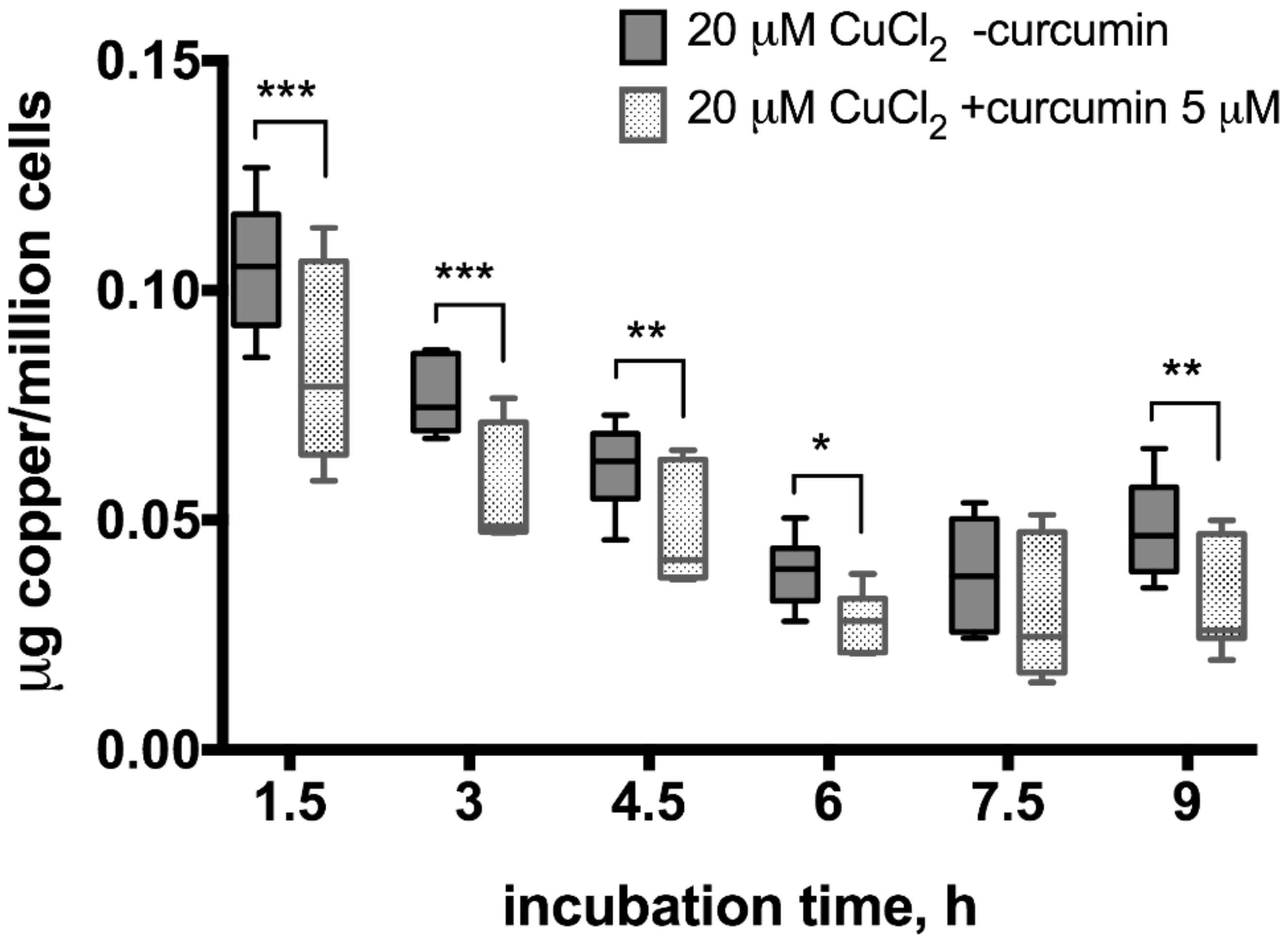
2.3. Atomic Absorption Spectrometry Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, H.C.; Lin, C.J.; Liu, W.J.; Jiang, R.R.; Jiang, Z.F. Dual effects of curcumin on neuronal oxidative stress in the presence of Cu(II). Food Chem. Toxicol. 2011, 49, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797s–811s. [Google Scholar] [PubMed]
- Roelofsen, H.; Wolters, H.; Van Luyn, M.J.; Miura, N.; Kuipers, F.; Vonk, R.J. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 2000, 119, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Camakaris, J. Menkes copper-translocating P-type ATPase (ATP7A): Biochemical and cell biology properties, and role in Menkes disease. J. Bioenerg. Biomembr. 2002, 34, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Coffey, A.J.; Durkie, M.; Hague, S.; McLay, K.; Emmerson, J.; Lo, C.; Klaffke, S.; Joyce, C.J.; Dhawan, A.; Hadzic, N.; et al. A genetic study of Wilson’s disease in the United Kingdom. Brain 2013, 136, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Rodo, M.; Gromadzka, G. Late onset Wilson’s disease: Therapeutic implications. Mov. Disord. 2008, 23, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Kegley, K.M.; Sellers, M.A.; Ferber, M.J.; Johnson, M.W.; Joelson, D.W.; Shrestha, R. Fulminant Wilson’s disease requiring liver transplantation in one monozygotic twin despite identical genetic mutation. Am. J. Transplant. 2010, 10, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Lombardo, M.F.; Ciriolo, M.R.; Rotilio, G. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem. Res. 2004, 29, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, J.; Pu, C.; Qiao, L.; Jiang, C. Wilson’s disease: A comprehensive review of the molecular mechanisms. Int. J. Mol. Sci. 2015, 16, 6419–6431. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Chan, K.M. PCR-cloning of tilapia ATP7A cDNA and its mRNA levels in tissues of tilapia following copper administrations. Aquat. Toxicol. 2011, 105, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Wijmenga, C.; Klomp, L.W. Molecular regulation of copper excretion in the liver. Proc. Nutr. Soc. 2004, 63, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef]
- Lee, J.; Pena, M.M.; Nose, Y.; Thiele, D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Prohaska, J.R.; Thiele, D.J. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6842–6847. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, E.M.; Klomp, L.W.; Merkx, M. Copper-dependent protein-protein interactions studied by yeast two-hybrid analysis. Biochem. Biophys. Res. Commun. 2004, 323, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Nyasae, L.K.; Schell, M.J.; Hubbard, A.L. Copper directs ATP7B to the apical domain of hepatic cells via basolateral endosomes. Traffic 2014, 15, 1344–1365. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.S.; Gitlin, J.D. Functional expression of the menkes disease protein reveals common biochemical mechanisms among the copper-transporting P-type ATPases. J. Biol. Chem. 1998, 273, 3765–3770. [Google Scholar] [CrossRef] [PubMed]
- Behari, M.; Pardasani, V. Genetics of Wilsons disease. Parkinsonism Relat. Disord. 2010, 16, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Siotto, M.; Bucossi, S.; Polimanti, R. In silico investigation of the ATP7B gene: Insights from functional prediction of non-synonymous substitution to protein structure. Biometals 2014, 27, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R. Copper subtype of Alzheimer’s disease (AD): Meta-analyses, genetic studies and predictive value of non-ceruloplasmim copper in mild cognitive impairment conversion to full, A.D. J. Trace Elem. Med. Biol. 2014, 28, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Beinhardt, S.; Leiss, W.; Stattermayer, A.F.; Graziadei, I.; Zoller, H.; Stauber, R.; Maieron, A.; Datz, C.; Steindl-Munda, P.; Hofer, H.; et al. Long-term outcomes of patients with Wilson disease in a large Austrian cohort. Clin. Gastroenterol. Hepatol. 2014, 12, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S. Modifying factors and phenotypic diversity in Wilson’s disease. Ann. N. Y. Acad. Sci. 2014, 1315, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Braiterman, L.; Nyasae, L.; Leves, F.; Hubbard, A.L. Critical roles for the COOH terminus of the Cu-ATPase ATP7B in protein stability, trans-Golgi network retention, copper sensing, and retrograde trafficking. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G69–G81. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Concilli, M.; Iacobacci, S.; Chesi, G.; Pastore, N.; Piccolo, P.; Paladino, S.; Baldantoni, D.; van IJzendoorn, S.C.; Chan, J.; et al. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell 2014, 29, 686–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altenburg, J.D.; Bieberich, A.A.; Terry, C.; Harvey, K.A.; Vanhorn, J.F.; Xu, Z.; Jo Davisson, V.; Siddiqui, R.A. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: Unique signaling not explained by the effects of either compound alone. BMC Cancer 2011, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nino, W.R.; Pedraza-Chaverri, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, S.; Li, W.; Guo, X.; Zhao, P.; Xu, J.; Chen, Y.; Pan, Q.; Liu, X.; Zychlinski, D.; et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson’s disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum. Mol. Genet. 2011, 20, 3176–3187. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Makino, T.; Takahara, K. Direct determination of plasma copper and zinc in infants by atomic absorption with discrete nebulization. Clin. Chem. 1981, 27, 1445–1447. [Google Scholar] [PubMed]
- Rothery, E. Analytical Methods for Graphite Tube Atomizers; Varian Australia Pty Ltd.: Mulgrave, Australia, 1988. [Google Scholar]
- Cater, M.A.; La Fontaine, S.; Shield, K.; Deal, Y.; Mercer, J.F. ATP7B mediates vesicular sequestration of copper: Insight into biliary copper excretion. Gastroenterology 2006, 130, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.H.; Suzuki, M.; Yamaguchi, Y.; Yuan, D.S.; Klausner, R.D.; Gitlin, J.D. Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1997, 272, 21461–21466. [Google Scholar] [CrossRef] [PubMed]
- Huster, D.; Hoppert, M.; Lutsenko, S.; Zinke, J.; Lehmann, C.; Mossner, J.; Berr, F.; Caca, K. Defective cellular localization of mutant ATP7B in Wilson’s disease patients and hepatoma cell lines. Gastroenterology 2003, 124, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Miura, N.; Kawarada, Y.; Terada, K.; Petrukhin, K.; Gilliam, T.; Sugiyama, T. Two forms of Wilson disease protein produced by alternative splicing are localized in distinct cellular compartments. Biochem. J. 1997, 326, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, P.V.; Stapelbroek, J.M.; Krieger, E.; de Bie, P.; van de Graaf, S.F.; de Groot, R.E.; van Beurden, E.; Spijker, E.; Houwen, R.H.; Berger, R.; et al. Reduced expression of ATP7B affected by Wilson disease-causing mutations is rescued by pharmacological folding chaperones 4-phenylbutyrate and curcumin. Hepatology 2009, 50, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Aston, N.S.; Watt, N.; Morton, I.E.; Tanner, M.S.; Evans, G.S. Copper toxicity affects proliferation and viability of human hepatoma cells (HepG2 line). Hum. Exp. Toxicol. 2000, 19, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jia, L.; Zhou, H.M.; Liu, Y.; Zhong, L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Strand, S.; Frank, N.; Knauft, J.; Wesch, H.; Galle, P.R.; Bartsch, H. Apoptosis and age-dependant induction of nuclear and mitochondrial etheno-DNA adducts in Long-Evans Cinnamon (LEC) rats: Enhanced DNA damage by dietary curcumin upon copper accumulation. Carcinogenesis 2005, 26, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Tizabi, Y.; Hurley, L.L.; Qualls, Z.; Akinfiresoye, L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules 2014, 19, 20864–20879. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzina, A.; Martinsone, I.; Svirskis, S.; Murovska, M.; Kalis, M. Curcumin Effect on Copper Transport in HepG2 Cells. Medicina 2018, 54, 14. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54020014
Berzina A, Martinsone I, Svirskis S, Murovska M, Kalis M. Curcumin Effect on Copper Transport in HepG2 Cells. Medicina. 2018; 54(2):14. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54020014
Chicago/Turabian StyleBerzina, Anita, Inese Martinsone, Simons Svirskis, Modra Murovska, and Martins Kalis. 2018. "Curcumin Effect on Copper Transport in HepG2 Cells" Medicina 54, no. 2: 14. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54020014




