Diagnostic Yield of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Cytological Smears and Cell Blocks: A Single-Institution Experience
Abstract
:1. Introduction
2. Experimental Section
Statistical Analysis
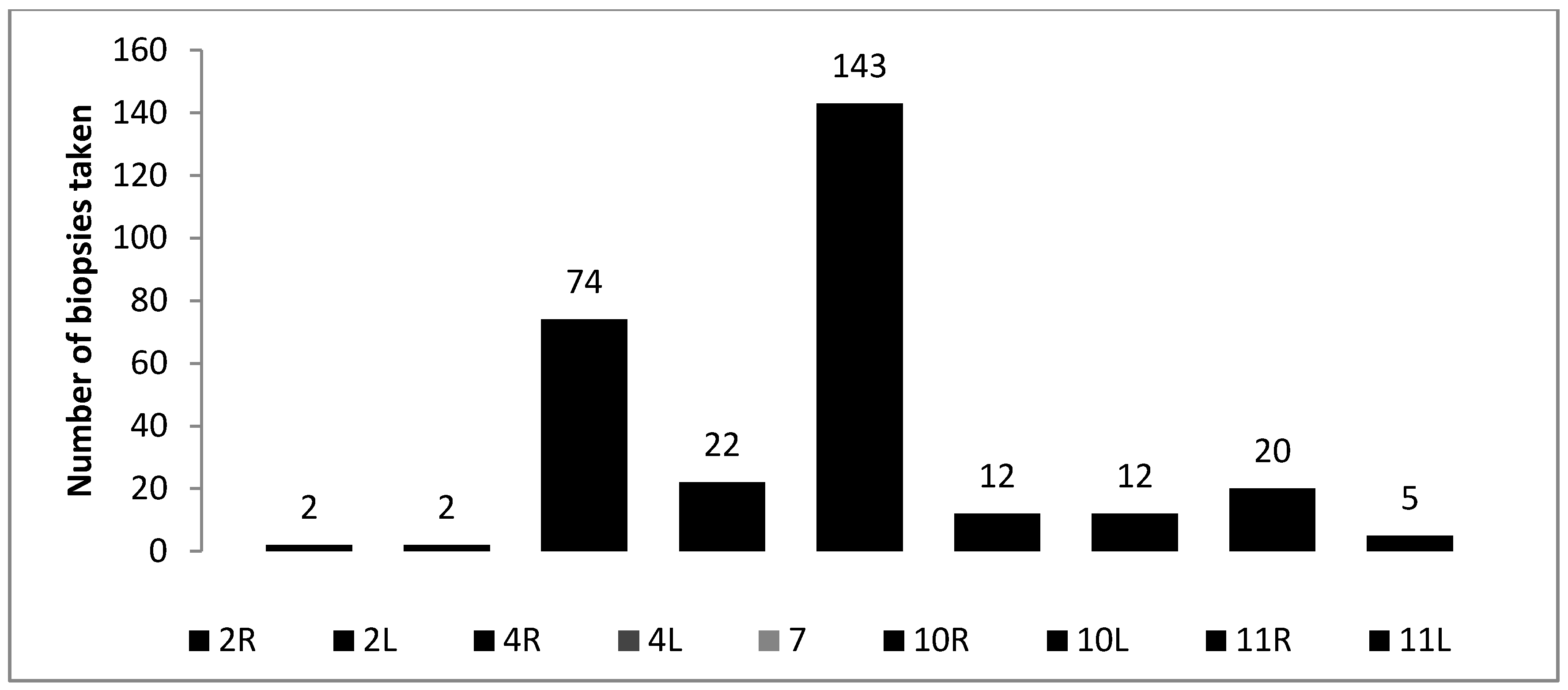
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herth, F.J.; Ernst, A. Endobronchial Ultrasound: An Atlas and Practical Guide; Springer: New York, NY, USA, 2009. [Google Scholar]
- Herth, F.J.; Krasnik, M.; Kahn, N.; Eberhardt, R.; Ernst, A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010, 138, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Jantz, M.A.; Wallace, M.; Vansteenkiste, J.; Silvestri, G.A. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines. Chest 2007, 132 (Suppl. 3), 202S–220S. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, K.; Chiyo, M.; Koh, E.; Moriya, Y.; Iyoda, A.; Sekine, Y.; Shibuya, K.; Iizasa, T.; Fujisawa, T. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005, 50, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Balamugesh, T.; Herth, F. Endobronchial ultrasound: A new innovation in bronchoscopy. Lung India 2009, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Annema, J.T.; Versteegh, M.I.; Veseliç, M.; Voigt, P.; Rabe, K.F. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J. Clin. Oncol. 2005, 23, 8357–8361. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Srinivasan, A.; Aggarwal, A.N.; Gupta, D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: A systematic review and meta-analysis. Respir. Med. 2012, 106, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yasufuku, K.; Fujiwara, T.; Chiyo, M.; Sekine, Y.; Shibuya, K.; Hiroshima, K.; Yoshino, I. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J. Thorac. Oncol. 2008, 3, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.P.; Jimenez, C.A.; Bruzzi, J.F.; Mhatre, A.D.; Lei, X.; Giles, F.J.; Fanning, T.; Morice, R.C.; Eapen, G.A. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008, 63, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Geake, J.; Hammerschlag, G.; Nguyen, P.; Wallbridge, P.; Jenkin, G.A.; Korman, T.M.; Jennings, B.; Johnson, D.F.; Irving, L.B.; Farmer, M.; et al. Utility of EBUS-TBNA for diagnosis of mediastinal tuberculous lymphadenitis: a multicentre Australian experience. J. Thorac. Dis. 2015, 7, 439. [Google Scholar] [PubMed]
- Herth, F.J.; Eberhardt, R.; Vilmann, P.; Krasnik, M.; Ernst, A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006, 61, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Plat, G.; Pierard, P.; Haller, A.; Hutsebaut, J.; Faber, J.; Dusart, M.; Eisendrath, P.; Sculier, J.P.; Ninane, V. Endobronchial ultrasound and positron emission tomography positive mediastinal lymph nodes. Eur. Respir. J. 2006, 27, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Kunzendorf, F.; Pfeifer, M.; Arzt, M.; Schulz, C. Endobronchial ultrasound-guided transbronchial needle aspiration in routine care-plenty of benign results and follow-up tests. Int. J. Clin. Pract. 2012, 66, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lema, L.; Fernandez-Villar, A.; Ruano-Ravina, A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur. Respir. J. 2009, 33, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Beasley, M.B.; Chitale, D.A.; Dacic, S.; Giaccone, G.; Jenkins, R.B.; Kwiatkowski, D.J.; Saldivar, J.S.; Squire, J.; et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J. Thorac. Oncol. 2013, 8, 823–859. [Google Scholar] [PubMed]
- Yasufuku, K.; Pierre, A.; Darling, G.; de Perrot, M.; Waddell, T.; Johnston, M.; da Cunha Santos, G.; Geddie, W.; Boerner, S.; Le, L.W.; et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J. Thorac. Cardiovasc. Surg. 2011, 142, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, K.; Chiyo, M.; Sekine, Y.; Chhajed, P.N.; Shibuya, K.; Iizasa, T.; Fujisawa, T. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004, 126, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, G.K.; Lee, H.S.; Kim, M.S.; Lee, J.M.; Kim, H.Y.; Nam, B.H.; Zo, J.I.; Hwangbo, B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: How many aspirations per target lymph node station? Chest 2008, 134, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Pascual, J.M.; Raimondo, M.; Woodward, T.A.; McComb, B.L.; Crook, J.E.; Johnson, M.M.; Al-Haddad, M.A.; Gross, S.A.; Pungpapong, S.; et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008, 299, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Pierre, A.F.; Nakajima, T.; de Perrot, M.; Darling, G.E.; Waddell, T.K.; Keshavjee, S.; Yasufuku, K. Endobronchial ultrasound-guided transbronchial needle aspiration in the management of previously treated lung cancer. Ann. Thorac. Surg. 2011, 92, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Nakajima, T.; Yasufuku, K.; Fujiwara, T.; Yoshida, S.; Suzuki, M.; Shibuya, K.; Hiroshima, K.; Nakatani, Y.; Yoshino, I. Lymph node staging by endobronchial ultrasound-guided transbronchial needle aspiration in patients with small cell lung cancer. Ann. Thorac. Surg. 2010, 90, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Mohan, A.; Ayub, I.I.; Jain, D.; Hadda, V.; Khilnani, G.C.; Guleria, R. Initial experience with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) from a tuberculosis endemic population. J. Bronchol. Interv. Pulmonol. 2014, 21, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Jernlås, B.; Nyberger, H.; Ek, L.; Öhman, R.; Jӧnsson, P.; Nozohoor, S. Diagnostic yield and efficacy of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal lymphadenopathy. Clin. Respir. J. 2012, 6, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sørhaug, S.; Hjelde, H.; Hatlen, P.; Olav Leira, H.; Salarinejad, M.; Nesvik, B.; Hollund, R.; Salarinejad, M.; Nesgård, K.; Nordhaug, D.O.; et al. Learning EBUS-TBNA-a 6-year experience at a single institution. Clin. Respir. J. 2016, 46. [Google Scholar] [CrossRef]
- Gu, P.; Zhao, Y.-Z.; Jiang, L.-Y.; Zhang, W.; Xin, Y.; Han, B.-H. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta-analysis. Eur. J. Cancer 2009, 45, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Nathan, N.A.; Narayan, E.; Smith, M.M.; Horn, M.J. Cell Block Cytology. Am. J. Clin. Pathol. 2000, 114, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Santos, J.; Serra, P.; Andreo, F.; Llatjós, M.; Castellà, E.; Monsó, E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer 2012, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D.; Yung, R.C.-W.; Burroughs, F.; Li, Q.K. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration. Cancer Cytopathol. 2009, 117, 482–490. [Google Scholar]
- Navani, N.; Lawrence, D.R.; Kolvekar, S.; Hayward, M.; McAsey, D.; Kocjan, G.; Falzon, M.; Capitanio, A.; Shaw, P.; Morris, S.; et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: A prospective trial. American journal of respiratory and critical care medicine. Am. Thoracic. Soc. 2012, 186, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, B.; Lee, G.-K.; Lee, H.S.; Lim, K.-Y.; Lee, S.-H.; Kim, H.-Y.; Lee, H.S.; Kim, M.S.; Lee, J.M.; Nam, B.H.; et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010, 138, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Shingyoji, M.; Nakajima, T.; Yoshino, M.; Yoshida, Y.; Ashinuma, H.; Itakura, M.; et al. Endobronchial Ultrasonography for Positron Emission Tomography and Computed Tomography-Negative Lymph Node Staging in Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2014, 98, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, E.; Seyhan, E.C.; Ozgul, A.; Gencoglu, A.; Ozgul, G.; Cam, E.; Kamiloglu, E. Efficacy of convex probe endobronchial ultrasound (CP-EBUS) assisted transbronchial needle aspiration for mediastinal staging in non-small cell lung cancer cases with mediastinal lymphadenopathy. Ann. Thorac. Cardiovasc. Surg. 2011, 17, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, A.; Shelley-Fraser, G.; Medford, A.R. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014, 19, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Yarmus, L.B.; Akulian, J.; Lechtzin, N.; Yasin, F.; Kamdar, B.; Ernst, A.; Ost, D.E.; Ray, C.; Greenhill, S.R.; Jimenez, C.A.; et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013, 143, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yasufuku, K.; Takahashi, R.; Shingyoji, M.; Hirata, T.; Itami, M.; Matsui, Y.; Itakura, M.; Iizasa, T.; Kimura, H. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011, 16, 90–94. [Google Scholar] [CrossRef] [PubMed]
| Cytology No (%) | Histology No (%) | Combined Evaluation No (%) | |
|---|---|---|---|
| Positive | 109 (50.7) | 115 (54.8) | 134 (62.3) |
| Negative | 67 (31.2) | 32 (15.2) | 57 (26.5) |
| Non-diagnostic | 39 (18.1) | 63 (30) | 24 (11.2) |
| Total | 215 (100) | 210 (100) | 215 (100) |
| Year | Sensitivity (%) | NPV (%) | Accuracy (%) | PPV (%) | Specificity (%) |
|---|---|---|---|---|---|
| 2009 | 68.8 | 56.5 | 77.8 | 100 | 100 |
| 2010 | 69.4 | 57.7 | 78.4 | 100 | 100 |
| 2011 | 95.5 | 66.7 | 95.8 | 100 | 100 |
| 2012 | 89.3 | 80.0 | 92.5 | 100 | 100 |
| 2013–2014 | 85.4 | 62.0 | 87.3 | 100 | 100 |
| Cytology No (%) | Histology No (%) | Combined Evaluation No (%) | |
|---|---|---|---|
| Lung cancer | 78 (72.9) | 78 (72.9) | 90 (84.1) |
| Metastatic cancer | 1 (33.3) | 2 (66.7) | 2 (66.7) |
| Sarcoidosis | 29 (55.8) | 34 (65.4) | 41 (78.8) |
| Tuberculosis | 1 (25) | 1 (25) | 1 (25) |
| Reactive lymphadenopathy | 32 (84.2) | 20 (52.6) | 33 (86.8) |
| Total | 141 (69.1) | 135 (67.8) | 167 (81.9) |
| Results of EBUS-TBNA | Lung Cancer (%) | Sarcoidosis (%) | p |
|---|---|---|---|
| Cytology | |||
| Sensitivity | 72.9 | 55.8 | 0.09 |
| NPV | 78.8 | 87.6 | 0.053 |
| Accuracy | 86.5 | 89.3 | NS |
| Specificity | 100 | 100 | NS |
| PPV | 100 | 100 | NS |
| Histology | |||
| Sensitivity | 72.9 | 65.4 | NS |
| NPV | 79.3 | 89.9 | 0.01 |
| Accuracy | 86.5 | 91.6 | NS |
| Specificity | 100 | 100 | NS |
| PPV | 100 | 100 | NS |
| Cytology and/or Histology | |||
| Sensitivity | 84.1 * | 78.8 ** | NS |
| NPV | 86.4 | 93.7 | 0.04 |
| Accuracy | 92.1 | 94.9 | NS |
| Specificity | 100 | 100 | NS |
| PPV | 100 | 100 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žemaitis, M.; Musteikienė, G.; Miliauskas, S.; Pranys, D.; Sakalauskas, R. Diagnostic Yield of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Cytological Smears and Cell Blocks: A Single-Institution Experience. Medicina 2018, 54, 19. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54020019
Žemaitis M, Musteikienė G, Miliauskas S, Pranys D, Sakalauskas R. Diagnostic Yield of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Cytological Smears and Cell Blocks: A Single-Institution Experience. Medicina. 2018; 54(2):19. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54020019
Chicago/Turabian StyleŽemaitis, Marius, Greta Musteikienė, Skaidrius Miliauskas, Darius Pranys, and Raimundas Sakalauskas. 2018. "Diagnostic Yield of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Cytological Smears and Cell Blocks: A Single-Institution Experience" Medicina 54, no. 2: 19. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54020019





