Alteration of Androgen Receptor Protein Stability by Triptolide in LNCaP Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Detection of Calcium Concentrations
2.4. Western Blot
2.5. Statistical Analysis
3. Results
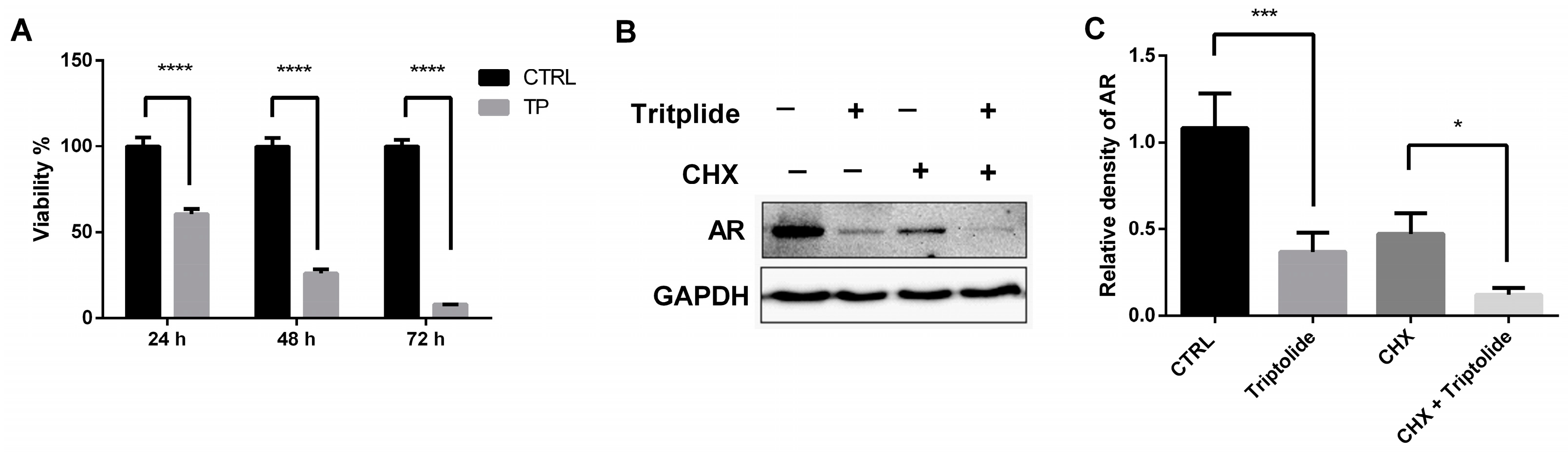
3.1. Triptolide Decreased the Stability of AR Protein
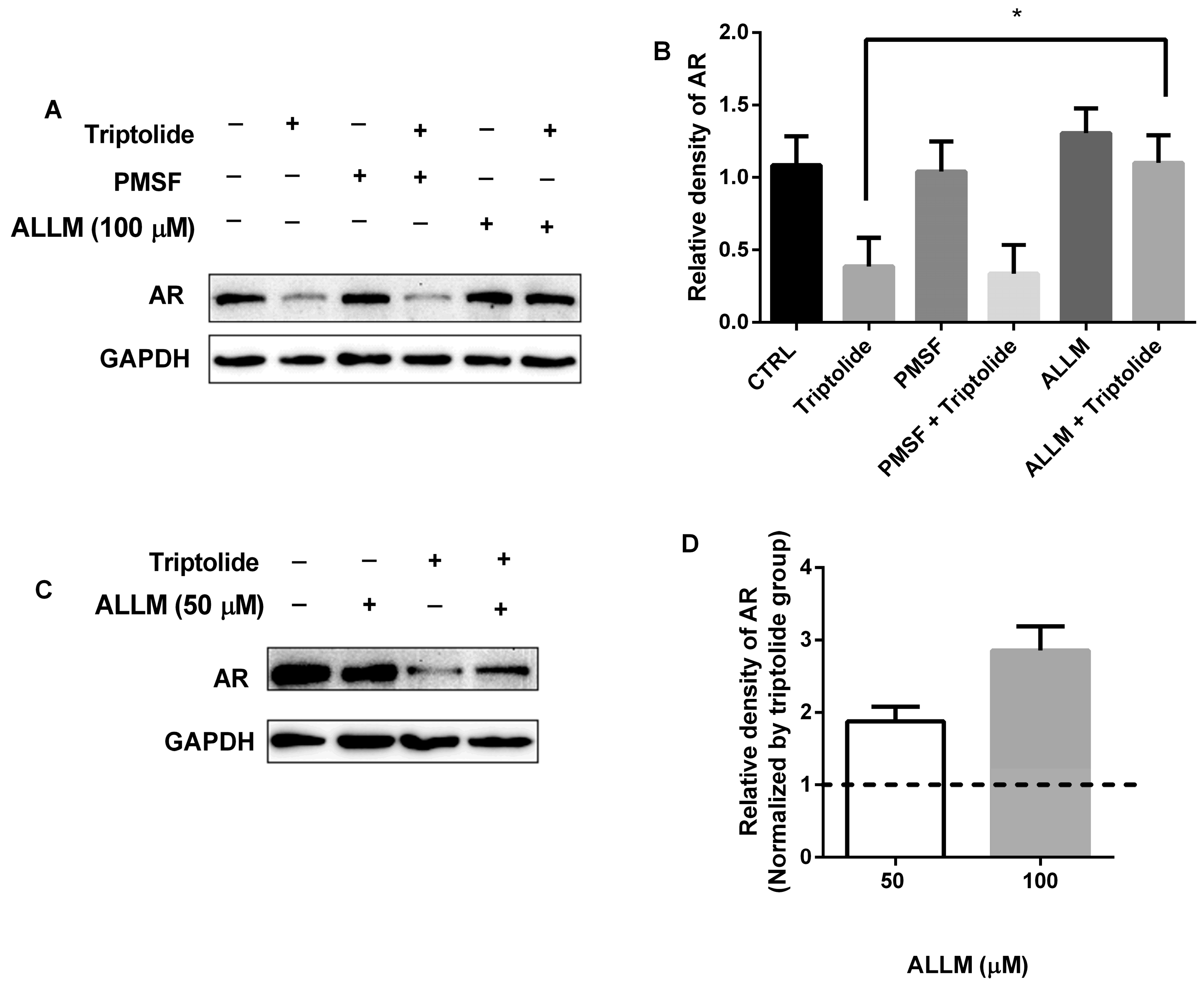
3.2. The Blocking of AR Protein Down-Regulation by Calpain Inhibitor, ALLM
3.3. No Effects of Triptolide on the Expression Levels of Calpains or Calpastatin Protein
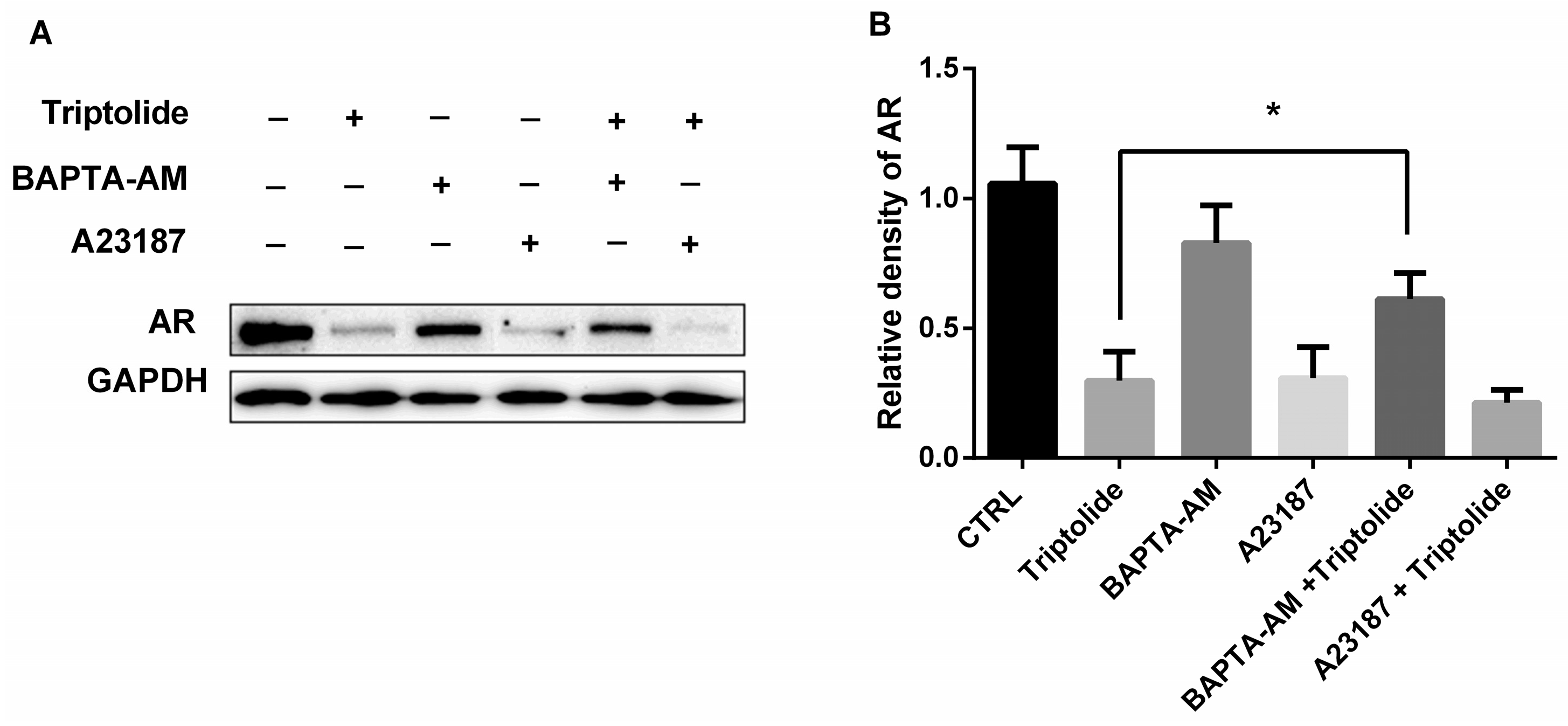
3.4. Increased Ca2+ Level in Triptolide Treated LNCaP Cells
3.5. The Effects of Calcium Regulator on AR Protein Down-Regulation by Triptolide
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer Statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N. Mechanisms of Androgen Receptor Activation in Castration-Resistant Prostate Cancer. Endocrinology 2013, 154, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, S.; Sarkar, F.H. Advances in Androgen Receptor Targeted Therapy for Prostate Cancer. J. Cell. Physiol. 2014, 229, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Linja, M.J.; Savinainen, K.J.; Saramaki, O.R.; Tammela, T.L.; Vessella, R.L.; Visakorpi, T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001, 61, 3550–3555. [Google Scholar] [PubMed]
- Edwards, J.; Krishna, N.S.; Grigor, K.M.; Bartlett, J.M. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 2003, 89, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Fidler, J.M.; Li, K.; Chung, C.; Wei, K.; Ross, J.A.; Gao, M.; Rosen, G.D. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol. Cancer Ther. 2003, 2, 855–862. [Google Scholar] [PubMed]
- Li, W.; Liu, Y.; Li, X.X.; Yu, Y.; Wu, J.J.; Wang, Q.; Huo, H.; Wang, L.M.; Yang, L. MAPKs are not involved in triptolide-induced cell growth inhibition and apoptosis in prostate cancer cell lines with different p53 status. Planta Med. 2011, 77, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kiviharju, T.M.; Lecane, P.S.; Sellers, R.G.; Peehl, D.M. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2666–2674. [Google Scholar]
- Huang, W.; He, T.; Chai, C.; Yang, Y.; Zheng, Y.; Zhou, P.; Qiao, X.; Zhang, B.; Liu, Z.; Wang, J.; et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS ONE 2012, 7, e37693. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.D.; Feng, Q.Q.; Gu, X.; Lu, D.; Li, W. Effects of triptolide on the expression of androgen receptor in human prostate LNCaP cells and its mechanism of action. Yao Xue Xue Bao (Acta Pharm. Sin.) 2015, 50, 1246–1251. [Google Scholar]
- Lee, D.H.; Goldberg, A.L. Proteasome inhibitors: Valuable new tools for cell biologists. Trends Cell Biol. 1998, 8, 397–403. [Google Scholar] [CrossRef]
- Lee, D.K.; Chang, C. Endocrine mechanisms of disease: Expression and degradation of androgen receptor: Mechanism and clinical implication. J. Clin. Endocrinol. Metab. 2003, 88, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Murthy, S.; Sarkar, F.H.; Sheng, S.; Reddy, G.P.; Dou, Q.P. Calpain-mediated androgen receptor breakdown in apoptotic prostate cancer cells. J. Cell. Physiol. 2008, 217, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Pelley, R.P.; Chinnakannu, K.; Murthy, S.; Strickland, F.M.; Menon, M.; Dou, Q.P.; Barrack, E.R.; Reddy, G.P.V. Calmodulin-androgen receptor (AR) interaction: Calcium-dependent, calpain-mediated breakdown of AR in LNCaP prostate cancer cells. Cancer Res. 2006, 66, 11754–11762. [Google Scholar] [CrossRef] [PubMed]
- Sivanandam, A.; Murthy, S.; Chinnakannu, K.; Bai, V.U.; Kim, S.H.; Barrack, E.R.; Menon, M.; Reddy, G.P.V. Calmodulin protects androgen receptor from calpain-mediated breakdown in prostate cancer cells. J. Cell. Physiol. 2011, 226, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Bi, X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016, 39, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Huang, W.; Zhang, Z.; Mao, L.; Han, Y.; Yan, J.; Lei, M. Triptolide induces protective autophagy through activation of the CaMKKbeta-AMPK signaling pathway in prostate cancer cells. Oncotarget 2016, 7, 5366–5382. [Google Scholar] [PubMed]
- Leuenroth, S.J.; Okuhara, D.; Shotwell, J.D.; Markowitz, G.S.; Yu, Z.; Somlo, S.; Crews, C.M. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2007, 104, 4389–4394. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Han, X.; Zeng, T.; Zhang, C.; Zou, C.; Xie, K. Changes in beclin-1 and micro-calpain expression in tri-ortho-cresyl phosphate-induced delayed neuropathy. Toxicol. Lett. 2012, 210, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Fidan, M.; Nowak, M.W.; Wilford, G.G.; Hogan, E.L.; Banik, N.L. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000, 852, 326–334. [Google Scholar] [CrossRef]
- Chen, H.; Libertini, S.J.; Wang, Y.; Kung, H.J.; Ghosh, P.; Mudryj, M. ERK regulates calpain 2-induced androgen receptor proteolysis in CWR22 relapsed prostate tumor cell lines. J. Biol. Chem. 2010, 285, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Wang, L.; Hu, Y.C.; Altuwaijri, S.; Chang, C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002, 21, 4037–4048. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Kanwar, J.; Schmitt, S.; Cui, Q.C.; Zhang, C.; Zhao, C.; Dou, Q.P. Inhibition of tumor cellular proteasome activity by triptolide extracted from the Chinese medicinal plant ‘thunder god vine’. Anticancer Res. 2011, 31, 1–10. [Google Scholar] [PubMed]
- LaFevre-Bernt, M.A.; Ellerby, L.M. Kennedy’s disease. Phosphorylation of the polyglutamine-expanded form of androgen receptor regulates its cleavage by caspase-3 and enhances cell death. J. Biol. Chem. 2003, 278, 34918–34924. [Google Scholar] [CrossRef] [PubMed]
- Mordente, J.A.; Choudhury, M.S.; Tazaki, H.; Mallouh, C.; Konno, S. Hydrolysis of Androgen Receptor by Cathepsin D: Its Biological Significance in Human Prostate Cancer. Br. J. Urol. 1998, 82, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kishi, M.; Saito, M.; Tanaka, T.; Higuchi, N.; Kominami, E.; Katunuma, N.; Murachi, T. Inhibitory Effect of Di- and Tripeptidyl Aldehydes on Calpains and Cathepsins. J. Enzyme Inhib. 1990, 3, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Blok, L.J.; Perry, J.E.; Lindzey, J.K.; Tindall, D.J. Calcium Regulation of Androgen Receptor Expression in the Human Prostate Cancer Cell Line LNCaP. Endocrinology 1995, 136, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, C.C.; Plymate, S.R. The Link between Androgen Receptor Splice Variants and Castration-Resistant Prostate Cancer. Horm. Cancer 2014, 5, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B.; Krueger, R.G.; Leedman, P.J. Differential Posttranscriptional Regulation of Androgen Receptor Gene Expression by Androgen in Prostate and Breast Cancer Cells. Endocrinology 1999, 140, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Schnellmann, R.G. Calpains, mitochondria, and apoptosis. Cardiovascular research. Cardiovasc. Res. 2012, 96, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Storr, S.J.; Thompson, N.; Pu, X.; Zhang, Y.; Martin, S.G. Calpain in Breast Cancer: Role in Disease Progression and Treatment Response. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2015, 82, 133–141. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.J.; Binge, L.C.; Sriratana, A.; Wang, H.; Robinson, P.A.; Pook, D.; Fedele, C.G.; Brown, S.; Dyson, J.M.; Cottle, D.L. Regulation of the transcriptional coactivator FHL2 licenses activation of the androgen receptor in castrate-resistant prostate cancer. Cancer Res. 2013, 73, 5066–5079. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Jaiswal, R.; Bebawy, M. Calcium-calpain Dependent Pathways Regulate Vesiculation in Malignant Breast Cells. Curr. Cancer Drug Targets 2017, 17, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Roseblade, A.; Luk, F.; Ung, A.; Bebawy, M. Targeting microparticle biogenesis: A novel approach to the circumvention of cancer multidrug resistance. Curr. Cancer Drug Targets 2015, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liu, B.-D.; Liao, K.; Liu, Y.; Wan, Z.-J.; Dong, Y.-F.; Cao, Q.-Q.; Zhu, Q.; Gu, X. Alteration of Androgen Receptor Protein Stability by Triptolide in LNCaP Cells. Medicina 2018, 54, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54030039
Li W, Liu B-D, Liao K, Liu Y, Wan Z-J, Dong Y-F, Cao Q-Q, Zhu Q, Gu X. Alteration of Androgen Receptor Protein Stability by Triptolide in LNCaP Cells. Medicina. 2018; 54(3):39. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54030039
Chicago/Turabian StyleLi, Wei, Bi-De Liu, Kai Liao, Yong Liu, Zi-Jin Wan, Yu-Fen Dong, Qian-Qian Cao, Qian Zhu, and Xiao Gu. 2018. "Alteration of Androgen Receptor Protein Stability by Triptolide in LNCaP Cells" Medicina 54, no. 3: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54030039



