Daily Cisplatin and Weekly Docetaxel versus Weekly Cisplatin Intra-Arterial Chemoradiotherapy for Late T2-3 Tongue Cancer: A Pilot and Feasibility Trial
Abstract
:1. Introduction
2. Materials and Methods
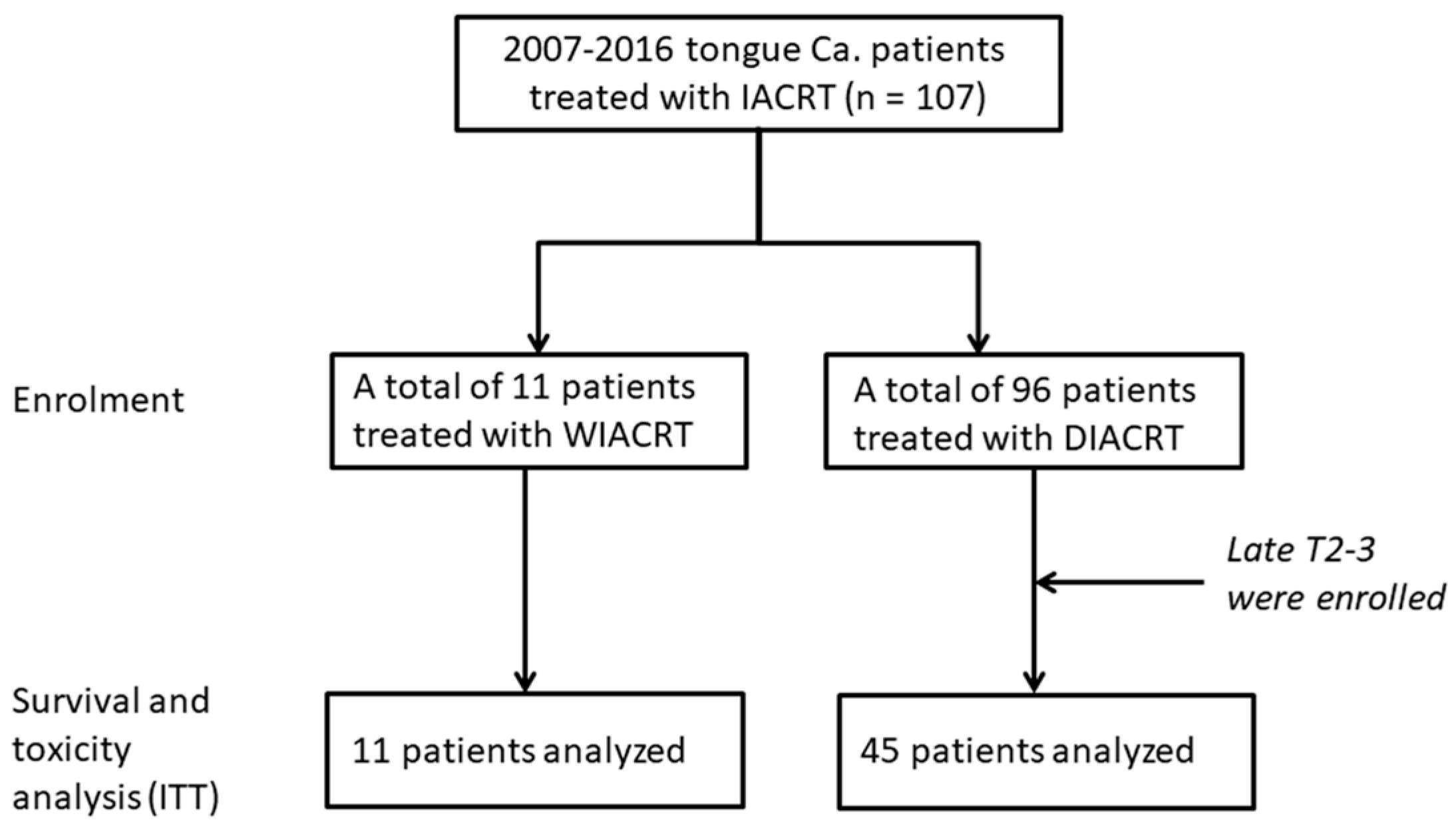
2.1. Study Design and Patients
2.2. Clinical Response Evaluation
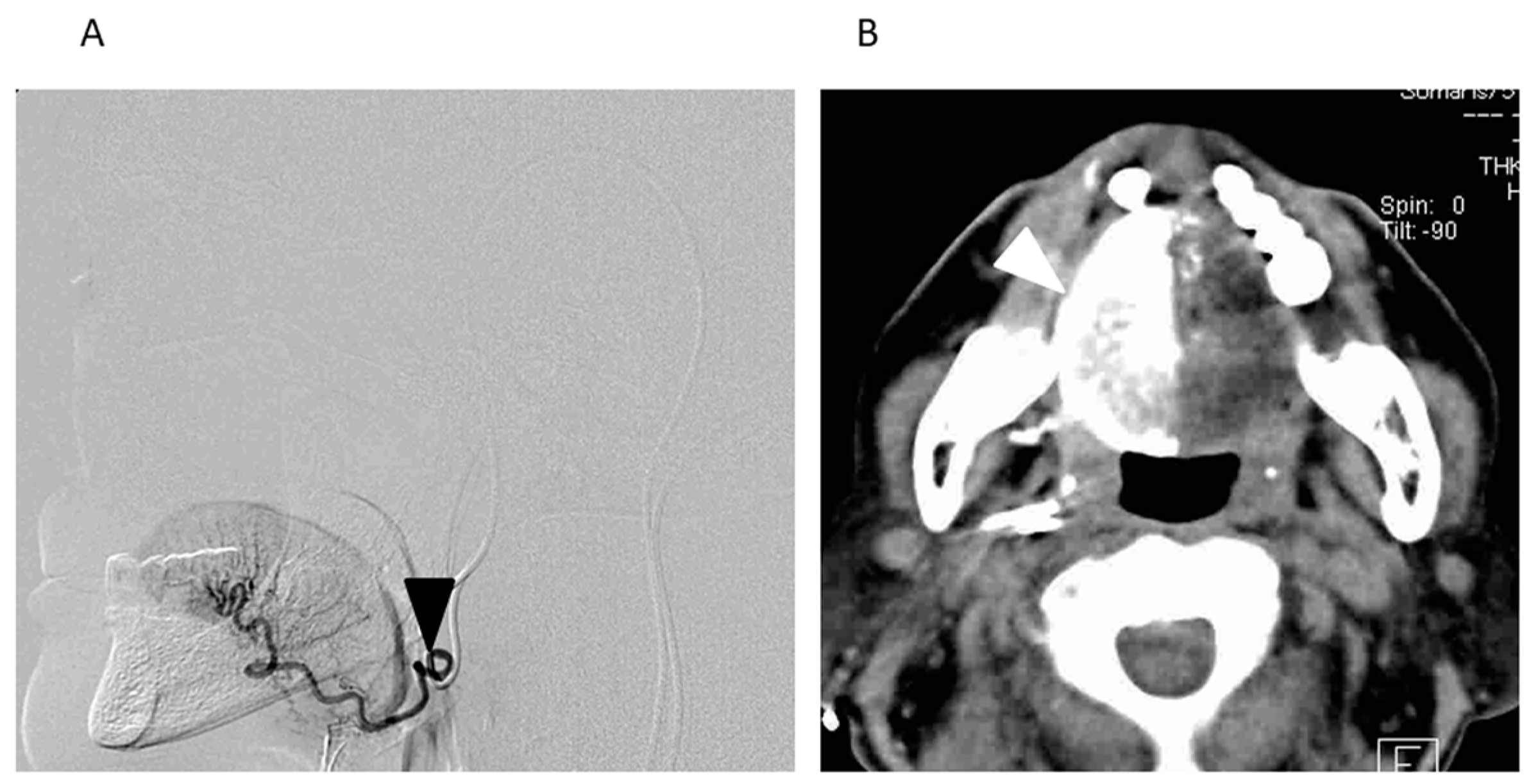
2.3. Radiotherapy
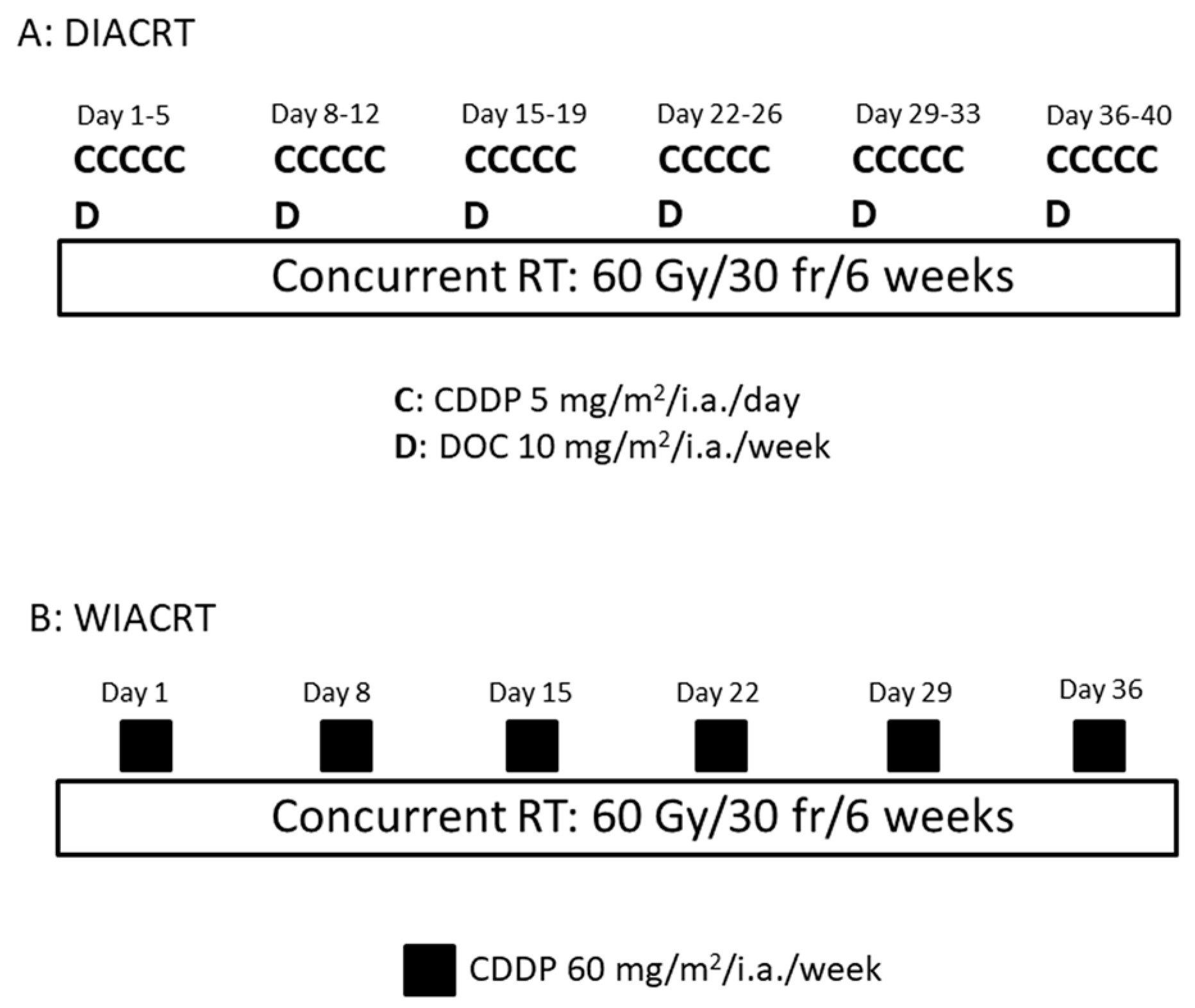
2.4. Chemotherapy
2.5. Evaluation of Toxicity
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Treatment Delivery
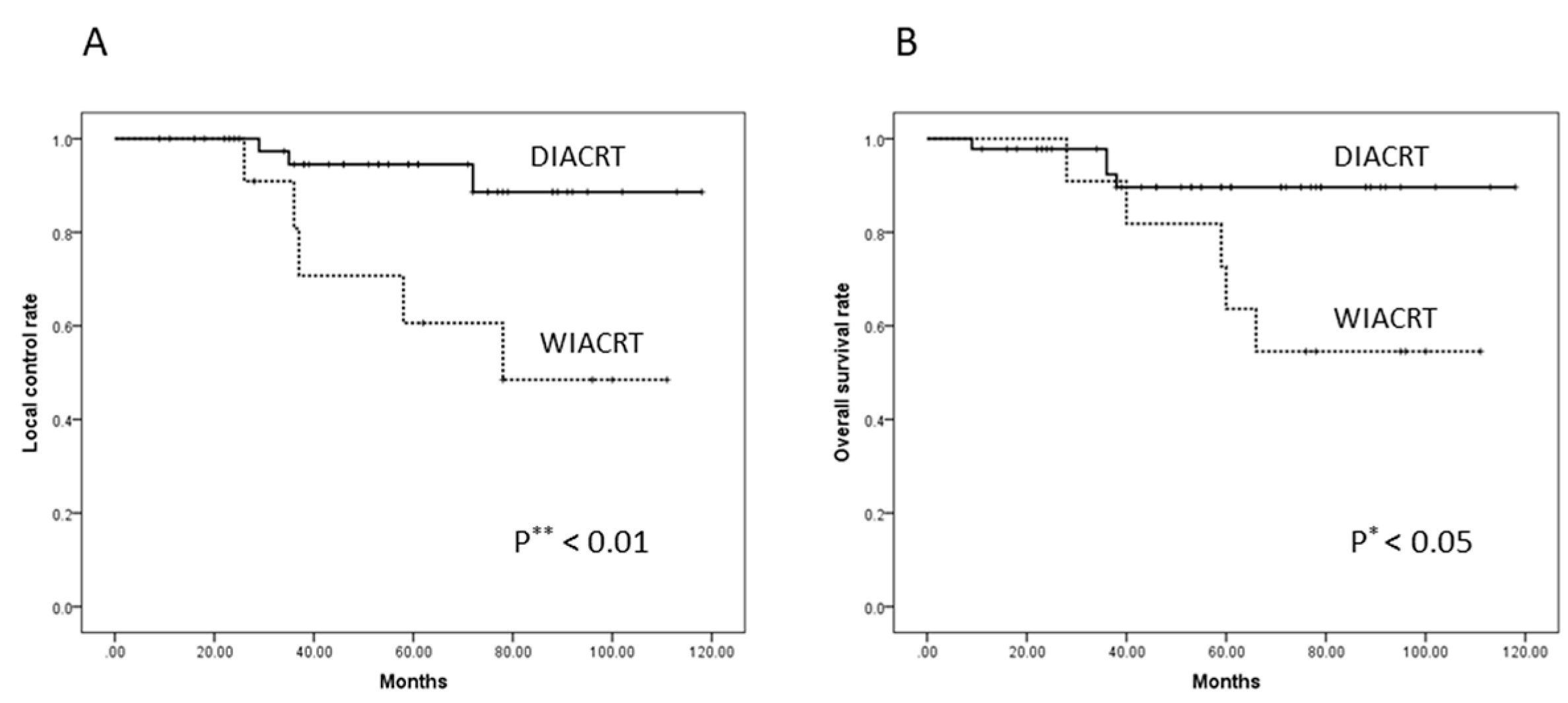
3.3. Response and Survival
3.4. Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robbins, K.T.; Storniolo, A.M.; Kerber, C.; Seagren, S.; Berson, A.; Howell, S.B. Rapid superselective high-dose cisplatin infusion for advanced head and neck malignancies. Head Neck 1992, 14, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Kumar, P.; Harris, J.; McCulloch, T.; Cmelak, A.; Sofferman, R.; Levine, P.; Weisman, R.; Wilson, W.; Weymuller, E.; et al. Supradose intra-arterial cisplatin and concurrent radiation therapy for the treatment of stage IV head and neck squamous cell carcinoma is feasible and efficacious in a multi-institutional setting: Results of Radiation Therapy Oncology Group Trial 9615. J. Clin. Oncol. 2005, 23, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Doweck, I.; Samant, S.; Vieira, F.; Kumar, P. Factors predictive of local disease control after intra-arterial concomitant chemoradiation (RADPLAT). Laryngoscope 2004, 114, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Storniolo, A.M.; Kerber, C.; Vicario, D.; Seagren, S.; Shea, M.; Hanchett, C.; Los, G.; Howell, S.B. Phase I study of highly selective supradose cisplatin infusions for advanced head and neck cancer. J. Clin. Oncol. 1994, 12, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Tohnai, I.; Fuwa, N.; Hayashi, Y.; Kaneko, R.; Tomaru, Y.; Hibino, Y.; Ueda, M. New superselective intra-arterial infusion via superficial temporal artery for cancer of the tongue and tumour tissue platinum concentration after carboplatin (CBDCA) infusion. Oral Oncol. 1998, 34, 387–390. [Google Scholar] [CrossRef]
- Mitsudo, K.; Koizumi, T.; Iida, M.; Iwai, T.; Nakashima, H.; Oguri, S.; Kioi, M.; Hirota, M.; Koike, I.; Hata, M.; et al. Retrograde superselective intra-arterial chemotherapy and daily concurrent radiotherapy for stage III and IV oral cancer: Analysis of therapeutic results in 112 cases. Radiother. Oncol. 2014, 111, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.E.; Day, N.E.; International Agency for Research on Cancer. Statistical Methods in Cancer Research. Vol. 1. The Analysis of Case-Control Studies; IARC Scientific Publication: Lyon, France, 1980; pp. 169–170. [Google Scholar]
- Iwai, T.; Fuwa, N.; Hirota, M.; Mitsudo, K.; Tohnai, I. Secure Surgical Method for Catheter Placement via the Occipital Artery to Achieve Retrograde Superselective Intra-Arterial Chemotherapy for Advanced Oral Cancer: Alternative to Approach via the Superficial Temporal Artery. Indian. J. Otolaryngol. Head Neck Surg. 2014, 66, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.T.; Whelan, R.D.H.; Shellard, S.A.; Mcclean, S.; Hosking, L.K.; Taxotere, D. Differential cytotoxic effects of docetaxel in a range of mammalian tumor cell lines and certain drug resistant sublines in vitro. Investig. New Drugs 1994, 12, 169–182. [Google Scholar] [CrossRef]
- Jeremic, B.; Shibamoto, Y.; Stanisavljevic, B.; Milojevic, L.; Milicic, B.; Nikolic, N. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: A prospective randomized trial. Radiother. Oncol. 1997, 43, 29–37. [Google Scholar] [CrossRef]
- Inohara, H.; Inoue, T.; Akahani, S.; Yamamoto, Y.; Takenaka, Y.; Nakagawa, T.; Isohashi, F.; Kubo, T. Concurrent chemoradiotherapy with cisplatin and docetaxel for advanced head and neck cancer. A phase I. study. Anticancer Res. 2004, 24, 4135–4140. [Google Scholar] [PubMed]
- Fuwa, N.; Kodaira, T.; Furutani, K.; Tachibana, H.; Nakamura, T. A new method of selective intra-arterial infusion therapy via the superficial temporal artery for head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Homma, A.; Inamura, N.; Oridate, N.; Suzuki, S.; Hatakeyama, H.; Mizumachi, T.; Kano, S.; Sakashita, T.; Onimaru, R.; Yasuda, K.; et al. Concomitant weekly cisplatin and radiotherapy for head and neck cancer. Jpn. J. Clin. Oncol. 2011, 41, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mohanti, B.K.; Thakar, A.; Bahadur, S.; Bhasker, S. Concomitant chemoradiation versus radical radiotherapy in advanced squamous cell carcinoma of oropharynx and nasopharynx using weekly cisplatin: A phase II randomized trial. Ann. Oncol. 2010, 21, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Newlin, H.E.; Amdur, R.J.; Riggs, C.E.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. Concomitant weekly cisplatin and altered fractionation radiotherapy in locally advanced head and neck cancer. Cancer 2010, 116, 4533–4540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traynor, A.M.; Richards, G.M.; Hartig, G.K.; Khuntia, D.; Cleary, J.F.; Wiederholt, P.A.; Bentzen, S.M.; Harari, P.M. Comprehensive IMRT plus weekly cisplatin for advanced head and neck cancer: The University of Wisconsin experience. Head Neck 2010, 32, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Lal, P.; Bajpai, R.; Goel, A.; Yadav, R.; Verma, M.; Kumar, S. Long term results of comparison of concurrent low-dose daily cisplatin versus the standard weekly cisplatin with six fractions per week radiotherapy in locally advanced head neck cancer. South Asian J. Cancer 2016, 5, 80–84. [Google Scholar] [PubMed]
- Rades, D.; Seidl, D.; Janssen, S.; Strojan, P.; Karner, K.; Bajrovic, A.; Hakim, S.G.; Wollenberg, B.; Schild, S.E. Comparing two lower-dose cisplatin programs for radio-chemotherapy of locally advanced head-and-neck cancers. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Sugiura, T.; Saikawa, Y.; Kubota, T.; Otani, Y.; Kumai, K.; Kitajima, M. Docetaxel enhances the cytotoxicity of cisplatin to gastric cells by modification of intracellular platinum metabolism. Cancer Sci. 2004, 95, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; Kuroiwa, T.; Tajima, T.; Tomita, K.; Ochiai, N.; Kawamoto, K. Efficacy of intra-arterial infusion therapy using a combination of cisplatin and docetaxel for recurrent head and neck cancers compared with cisplatin alone. Clin. Oncol. 2003, 15, 467–472. [Google Scholar] [CrossRef]
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer-surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Chaw, C.; Kovarik, J.; Aslam, S.; Jackson, A.; Kelly, J.; Dobrowsky, W.; Kelly, C. Primary Concurrent Chemoradiation in Head and Neck Cancers with Weekly Cisplatin Chemotherapy: Analysis of Compliance, Toxicity and Survival. Int. Arch. Otorhinolaryngol. 2017, 21, 171–177. [Google Scholar] [PubMed]
- Fuwa, N.; Kodaira, T.; Furutani, K.; Tachibana, H.; Nakamura, T.; Nakahara, R.; Tomoda, T.; Inokuchi, H.; Daimon, T. Intra-arterial chemoradiotherapy for locally advanced oral cavity cancer: Analysis of therapeutic results in 134 cases. Br. J. Cancer 2008, 98, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tada, Y.; Maruya, S.; Takeishi, E.; Miura, K.; Masubuchi, T.; Fushimi, C.; Hasegawa, H.; Kamata, S. Intra-arterial chemoradiation therapy with weekly low-dose cisplatin for squamous cell carcinoma of the maxillary sinus. Int. J. Oral Maxillofac. Surg. 2015, 44, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, N.; Kodaira, T.; Furutani, K.; Tachibana, H.; Nakamura, T.; Nakahara, R.; Tomoda, T.; Inokuti, H.; Daimon, T. Arterial Chemoradiotherapy for Locally Advanced Tongue Cancer: Analysis of Retrospective Study of Therapeutic Results in 88 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Nakamura, T.; Takada, A.; Makita, C.; Suzuki, M.; Azami, Y.; Kato, T.; Hayashi, Y.; Ono, T.; Toyomasu, Y.; et al. Treatment results of alternating chemoradiotherapy followed by proton beam therapy boost combined with intra-arterial infusion chemotherapy for stage III-IVB tongue cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, A.; Barth, E.L.; Kokemueller, H.; Wegener, G. Recurrent carcinoma of the head and neck: Treatment strategies and survival analysis in a 20-year period. Oral Oncol. 2004, 40, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Mitsudo, K.; Hayashi, Y.; Minamiyama, S.; Ohhashi, N.; Iida, M.; Iwai, T.; Oguri, S.; Koizumi, T.; Kioi, M.; Hirota, M.; et al. Chemoradiotherapy using retrograde superselective intra-arterial infusion for tongue cancer: Analysis of therapeutic results in 118 cases. Oral Oncol. 2018, 79, 71–77. [Google Scholar] [CrossRef] [PubMed]
| Treatment Type | ||||
|---|---|---|---|---|
| Characteristics | Total No. | DIACRT | WIACRT | p Value |
| No. of patients | 56 | 45 | 11 | |
| Median age, years (range) | 59 (36–78) | 60 (36–72) | 59 (40–78) | 0.332 |
| Gender | ||||
| Male | 29 | 22 | 7 | 0.612 |
| Female | 27 | 23 | 4 | |
| Performance status (ECOG) | ||||
| 0 | 53 | 43 | 10 | 0.787 |
| 1 | 3 | 2 | 1 | |
| T classification | ||||
| Late T2 | 34 | 25 | 9 | 0.392 |
| T3 | 22 | 20 | 2 | |
| N classification | ||||
| N0 | 42 | 34 | 8 | 0.215 |
| N1 | 14 | 11 | 3 | |
| Stage | ||||
| II | 27 | 20 | 7 | 0.553 |
| III | 29 | 25 | 4 | |
| Tumor differentiation | ||||
| Low grade | 5 | 4 | 1 | 0.275 |
| Moderate grade | 23 | 19 | 4 | |
| High grade | 28 | 22 | 6 | |
| Treatment delivery | ||||
| Median RT dose (Gy) (range) | 60 (50–70) | 60 (4–60) | 0.422 | |
| Cumulative dose of CDDP (mg/m2) (range) | 150 (135–175) | 360 (60–360) | 0.0023 | |
| DIACRT (n = 45) | WIACRT (n = 11) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Toxicities | G 2 | G 3 | G 4 | G 5 | G 2 | G 3 | G 4 | G 5 | p Value |
| Acute | |||||||||
| Neutropenia | 8 | 3 | 0 | 0 | 4 | 1 | 0 | 0 | 0.213 |
| Thrombocytopenia | 8 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.665 |
| Anemia | 8 | 3 | 0 | 0 | 4 | 1 | 0 | 0 | 0.112 |
| AKI | 3 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0.364 |
| Nausea/vomiting | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0.153 |
| CRI | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0.711 |
| FN | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0.104 |
| Mucositis | 13 | 32 | 0 | 0 | 5 | 5 | 0 | 0 | 0.222 |
| Dermatitis | 28 | 11 | 0 | 0 | 5 | 4 | 0 | 0 | 0.412 |
| Dysphagia | 15 | 30 | 0 | 0 | 5 | 5 | 0 | 0 | 0.109 |
| Late | |||||||||
| xerostomia | 13 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0.193 |
| ORN | 9 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0.207 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Minamiyama, S.; Ohya, T.; Iida, M.; Iwai, T.; Koizumi, T.; Oguri, S.; Hirota, M.; Kioi, M.; Hata, M.; et al. Daily Cisplatin and Weekly Docetaxel versus Weekly Cisplatin Intra-Arterial Chemoradiotherapy for Late T2-3 Tongue Cancer: A Pilot and Feasibility Trial. Medicina 2018, 54, 52. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54040052
Hayashi Y, Minamiyama S, Ohya T, Iida M, Iwai T, Koizumi T, Oguri S, Hirota M, Kioi M, Hata M, et al. Daily Cisplatin and Weekly Docetaxel versus Weekly Cisplatin Intra-Arterial Chemoradiotherapy for Late T2-3 Tongue Cancer: A Pilot and Feasibility Trial. Medicina. 2018; 54(4):52. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54040052
Chicago/Turabian StyleHayashi, Yuichiro, Shuhei Minamiyama, Takashi Ohya, Masaki Iida, Toshinori Iwai, Toshiyuki Koizumi, Senri Oguri, Makoto Hirota, Mitomu Kioi, Masaharu Hata, and et al. 2018. "Daily Cisplatin and Weekly Docetaxel versus Weekly Cisplatin Intra-Arterial Chemoradiotherapy for Late T2-3 Tongue Cancer: A Pilot and Feasibility Trial" Medicina 54, no. 4: 52. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54040052





