Impact of Left Ventricular Diastolic Dysfunction and Biomarkers on Pulmonary Hypertension in Patients with Severe Aortic Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Transthoracic Echocardiography
2.3. Blood Sampling
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carabello, B.A. Introduction to aortic stenosis. Circ. Res. 2013, 113, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromso study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeder, M.T.; Weber, L.; Buser, M.; Gerhard, M.; Haager, P.K.; Maisano, F.; Rickli, H. Pulmonary hypertension in aortic and mitral valve disease. Front. Cardiovasc. Med. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.J.; Wenaweser, P.; Ceylan, O.; Rat-Wirtzler, J.; Stortecky, S.; Heg, D.; Spitzer, E.; Zanchin, T.; Praz, F.; Tuller, D.; et al. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: Insights from the new proposed pulmonary hypertension classification. Circ. Cardiovasc. Interv. 2015, 8, e002358. [Google Scholar] [CrossRef] [PubMed]
- Lucon, A.; Oger, E.; Bedossa, M.; Boulmier, D.; Verhoye, J.P.; Eltchaninoff, H.; Iung, B.; Leguerrier, A.; Laskar, M.; Leprince, P.; et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: Study from the France 2 registry. Circ. Cardiovasc. Interv. 2014, 7, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Roselli, E.E.; Abdel Azim, A.; Houghtaling, P.L.; Jaber, W.A.; Blackstone, E.H. Pulmonary hypertension is associated with worse early and late outcomes after aortic valve replacement: Implications for transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2012, 144, 1067–1074.e1062. [Google Scholar] [CrossRef] [PubMed]
- Carnero-Alcazar, M.; Reguillo-Lacruz, F.; Alswies, A.; Villagran-Medinilla, E.; Maroto-Castellanos, L.C.; Rodriguez-Hernandez, J. Short- and mid-term results for aortic valve replacement in octogenarians. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 549–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinning, J.M.; Wollert, K.C.; Sedaghat, A.; Widera, C.; Radermacher, M.C.; Descoups, C.; Hammerstingl, C.; Weber, M.; Stundl, A.; Ghanem, A.; et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am. Heart J. 2015, 170, 821–829. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.P.; Guerrero, M.; Thourani, V.H.; Kodali, S.; Heldman, A.; Williams, M.; Xu, K.; Pichard, A.; Mack, M.; Babaliaros, V.; et al. Prognostic value of serial b-type natriuretic peptide measurement in transcatheter aortic valve replacement (from the partner trial). Am. J. Cardiol. 2015, 115, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Furer, A.; Lindman, B.R.; Burkhoff, D.; Marquis-Gravel, G.; Francese, D.P.; Ben-Yehuda, O.; Pibarot, P.; Gillam, L.D.; Leon, M.B.; et al. Biomarkers in aortic stenosis: A systematic review. Struct. Heart 2017, 1, 18–30. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): Endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Alushi, B.; Beckhoff, F.; Leistner, D.; Franz, M.; Reinthaler, M.; Stahli, B.E.; Morguet, A.; Figulla, H.R.; Doenst, T.; Maisano, F.; et al. Pulmonary hypertension in patients with severe aortic stenosis: Prognostic impact after transcatheter aortic valve replacement: Pulmonary hypertension in patients undergoing TAVR. JACC Cardiovasc. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Chang, S.A.; Kim, H.K.; Kim, S.J.; Lee, S.P.; Park, S.J.; Kim, Y.J.; Cho, G.Y.; Sohn, D.W.; Oh, J.K. Determinants of pulmonary hypertension development in moderate or severe aortic stenosis. Int. J. Cardiovasc. Imaging 2014, 30, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, P.; Antonini-Canterin, F.; Ribichini, F.; D’Aloia, A.; Ferrero, V.; Cervesato, E.; Pavan, D.; Burelli, C.; Nicolosi, G. Pulmonary artery hypertension in adult patients with symptomatic valvular aortic stenosis. Am. J. Cardiol. 2000, 85, 204–208. [Google Scholar] [CrossRef]
- Buonanno, C.; Johnson, L.W.; Bowser, M.A.; Hapanowicz, M.B.; Marvasti, M.; Parker, F.B. Pulmonary hypertension in severe aortic stenosis. Incidence, mechanisms, clinical and surgical implications. G. Ital. Di Cardiol. 1987, 17, 636–641. [Google Scholar]
- Bruch, C.; Stypmann, J.; Grude, M.; Gradaus, R.; Breithardt, G.; Wichter, T. Tissue doppler imaging in patients with moderate to severe aortic valve stenosis: Clinical usefulness and diagnostic accuracy. Am. Heart J. 2004, 148, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Gertz, Z.M.; O’Donnell, W.T.; Herrmann, H.C.; Forfia, P.R. Pulmonary hypertension is a manifestation of congestive heart failure and left ventricular diastolic dysfunction in octogenarians with severe aortic stenosis. Pulm. Circ. 2015, 5, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Casaclang-Verzosa, G.; Nkomo, V.T.; Sarano, M.E.; Malouf, J.F.; Miller, F.A., Jr.; Oh, J.K. E/EA is the major determinant of pulmonary artery pressure in moderate to severe aortic stenosis. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2008, 21, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Varadarajan, P.; Pai, R.G. Echocardiographic predictors of pulmonary hypertension in patients with severe aortic stenosis. Eur. J. Echocardiogr. J. Work. Group Echocardiogr. Eur. Soc. Cardiol. 2008, 9, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Wentworth, B.; Choudhary, R.; Landa Ade, L.; Kipper, B.; Fard, A.; Maisel, A.S. Cardiac biomarkers: New tools for heart failure management. Cardiovasc. Diagn. Ther. 2012, 2, 147–164. [Google Scholar] [PubMed]
- Casserly, B.; Klinger, J.R. Brain natriuretic peptide in pulmonary arterial hypertension: Biomarker and potential therapeutic agent. Drug Des. Dev. Ther. 2009, 3, 269–287. [Google Scholar]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T. Growth differentiation factor 15 in heart failure: An update. Curr. Heart Fail. Rep. 2012, 9, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Kempf, T.; Rector, T.S.; Tapken, H.; Allhoff, T.; Jantzen, F.; Kuskowski, M.; Cohn, J.N.; Drexler, H.; Wollert, K.C. Serial measurement of growth-differentiation factor-15 in heart failure: Relation to disease severity and prognosis in the valsartan heart failure trial. Circulation 2010, 122, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Heringlake, M.; Charitos, E.I.; Gatz, N.; Kabler, J.H.; Beilharz, A.; Holz, D.; Schon, J.; Paarmann, H.; Petersen, M.; Hanke, T. Growth differentiation factor 15: A novel risk marker adjunct to the Euroscore for risk stratification in cardiac surgery patients. J. Am. Coll. Cardiol. 2013, 61, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Peter, T.; Olofsson, S.; James, S.; Johnston, N.; Lindahl, B.; Horn-Wichmann, R.; Brabant, G.; Simoons, M.L.; et al. Prognostic value of growth-differentiation factor-15 in patients with non-st-elevation acute coronary syndrome. Circulation 2007, 115, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Krau, N.C.; Lunstedt, N.S.; Freitag-Wolf, S.; Brehm, D.; Petzina, R.; Lutter, G.; Bramlage, P.; Dempfle, A.; Frey, N.; Frank, D. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur. J. Heart Fail. 2015, 17, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, A.; Magnino, C.; Veglio, F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2010, 23, 225–239; quiz 332–334. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 60) | PSP < 45 mmHg (n = 47) | PSP ≥ 45 mmHg (n = 13) | p Value | |
|---|---|---|---|---|
| Age, years | 69 ± 9 | 68 ± 9 | 72 ± 10 | 0.591 |
| Sex, male n (%) | 60 (50) | 25 (53.2) | 5 (38.5) | 0.347 |
| Glomerulal filtration rate, mL/min/1.732 | 84.5 ± 31.4 | 88.9 ± 33.3 | 68.5 ± 15.4 | 0.070 |
| Body mass index, kg/m2 | 28.6 ± 4.9 | 28.8 ± 5.0 | 27.9 ± 4.5 | 0.716 |
| Arterial hypertension, n (%) | 48 (80) | 38 (80.9) | 10 (76.9) | 0.754 |
| Diabetes mellitus, n (%) | 5 (8.3) | 5 (10.6) | 0 (0) | 0.219 |
| NYHA functional class 3–4, n (%) | 26 (43.3) | 19 (40.5) | 7 (53.9) | 0.449 |
| Aortic valve area, cm2 | 0.81 ± 0.19 | 0.81 ± 0.19 | 0.79 ± 0.21 | 0.829 |
| Mitral regurgitation mild, n (%) | 60 (100) | 47 (100) | 13 (100) | 0.853 |
| LV end-diastolic diameter index, mm/m2 | 25.9 ± 3.7 | 24.1 ± 3.9 | 27.7 ± 2.8 | 0.05 |
| LV Mass index, g/m2 | 132.2 ± 36.9 | 129.3 ± 34.9 | 142.69 ± 43.22 | 0.374 |
| LV EF, % | 50 ± 8 | 50 ± 8 | 49 ± 8 | 0.588 |
| LA volume, mL | 95.4 ± 29.7 | 90.1 ± 25.7 | 114.8 ± 35.9 | 0.010 |
| PSP, mmHg | 40.5 ± 11.6 | 35.5 ± 4.8 | 58.5 ± 11.2 | 0.001 |
| E/A ratio | 1.1 ± 0.5 | 0.9 ± 0.4 | 1.6 ± 0.8 | 0.002 |
| E/E‘ ratio | 14.8 ± 6.3 | 13.8± 5.4 | 18.7 ± 8.2 | 0.047 |
| MV DT, ms | 226.4 ± 53.9 | 235.7 ± 50.4 | 192.7 ± 54.5 | 0.014 |
| NT-proBNP, ng/L | 730 (379–2758.5) | 602 (366–1818) | 4916 (394–12,032) | 0.049 |
| GDF-15, pg/L | 3457.9 ± 662.4 | 3159.2 ± 1568.7 | 4525.0 ± 1653.8 | 0.030 |
| Parameters/Threshold | β | SE | Chi-Square | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| E/E’ ratio > 14 | 1.79 | 0.73 | 6.74 | 6.00 | 1.41–25.48 | 0.009 |
| DT ≤ 177.5 ms | 2.2 | 0.76 | 8.76 | 9.31 | 2.06–41.14 | 0.001 |
| LA volume > 100 mL | 2.27 | 0.82 | 10.23 | 9.70 | 1.92–49.03 | 0.002 |
| NT-proBNP > 4060 ng/L | 2.52 | 0.76 | 11.86 | 12.54 | 2.80–55.99 | 0.001 |
| GDF-15 > 3393 pg/mL | 2.90 | 1.03 | 9.26 | 18.33 | 2.39–140.39 | 0.001 |
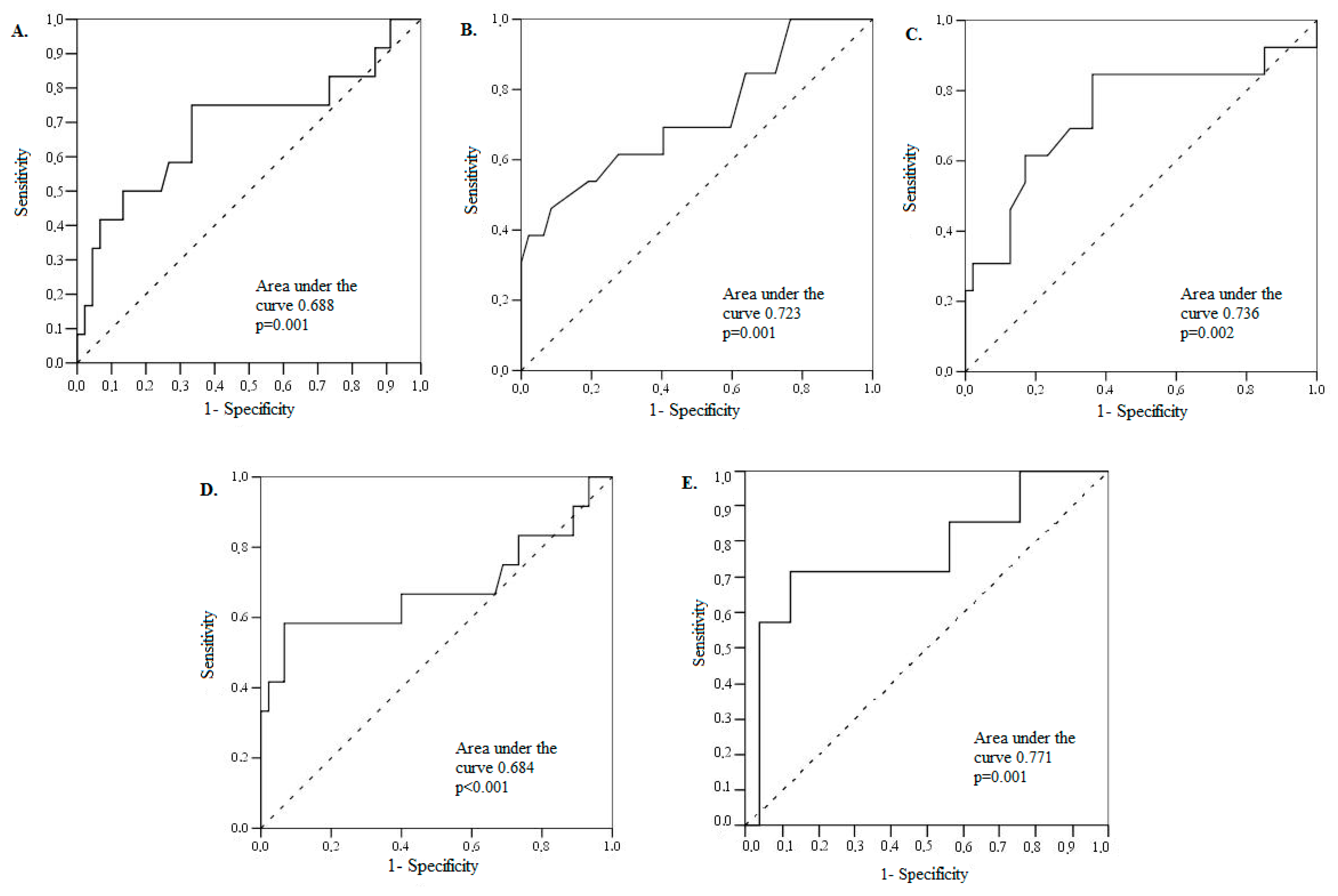
| Parameters/Threshold | Area under Curve | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | p Value |
|---|---|---|---|---|---|---|---|
| E/E’ ratio > 14 | 68.8 | 49.3–88.2 | 75.0 | 66.7 | 37 | 90 | 0.009 |
| DT ≤ 177.5 ms | 76.2 | 59.8–92.7 | 46.2 | 91.5 | 39 | 93 | 0.001 |
| LA volume > 100 mL | 73.6 | 55.8–91.4 | 84.6 | 63.8 | 60 | 86 | 0.002 |
| NT-proBNP > 4060 ng/L | 68.4 | 47.0–89.8 | 58.3 | 93.3 | 70 | 89 | <0.001 |
| GDF-15 > 3393 pg/mL | 77.1 | 54.7–99.6 | 71.4 | 88.0 | 62 | 92 | 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumauskienė, B.; Krivickienė, A.; Jonkaitienė, R.; Vaškelytė, J.J.; Siudikas, A.; Ereminienė, E. Impact of Left Ventricular Diastolic Dysfunction and Biomarkers on Pulmonary Hypertension in Patients with Severe Aortic Stenosis. Medicina 2018, 54, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54040063
Gumauskienė B, Krivickienė A, Jonkaitienė R, Vaškelytė JJ, Siudikas A, Ereminienė E. Impact of Left Ventricular Diastolic Dysfunction and Biomarkers on Pulmonary Hypertension in Patients with Severe Aortic Stenosis. Medicina. 2018; 54(4):63. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54040063
Chicago/Turabian StyleGumauskienė, Birutė, Aušra Krivickienė, Regina Jonkaitienė, Jolanta Justina Vaškelytė, Adakrius Siudikas, and Eglė Ereminienė. 2018. "Impact of Left Ventricular Diastolic Dysfunction and Biomarkers on Pulmonary Hypertension in Patients with Severe Aortic Stenosis" Medicina 54, no. 4: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54040063





