Cardiac Glucolipotoxicity and Cardiovascular Outcomes
Abstract
:1. Introduction
2. Cardiac Development, Bioenergetics, and Physiology
3. Postnatal Cardiac Insulin Signaling and Insulin Resistance
4. Postnatal Cardiac Fatty Acid Signaling
5. High Fat Diets and Fatty Acids Alter Cardiac Gene Expression Involved in Hypertrophy
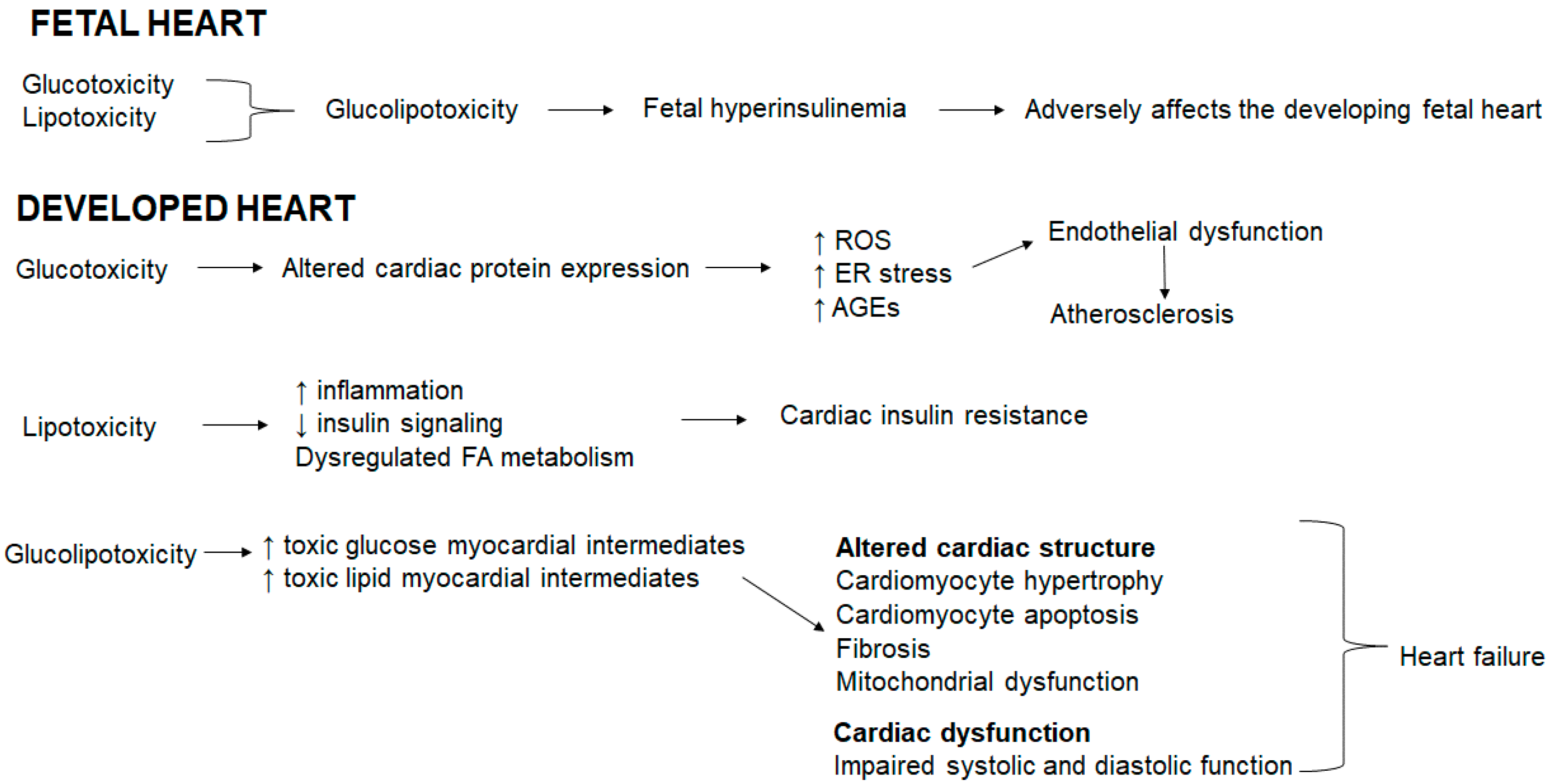
6. Cardiac Glucolipotoxicity
6.1. Cardiac Bioenergetics in Cardiovascular Disease
6.2. Diabetes and Cardiovascular Disease
6.3. The Obesity Paradox
6.4. Cardiac Lipotoxicity and Cardioprotection
6.5. Gluco-, Lipo- and Glucolipotoxicity
6.6. Glucolipotoxicity Shapes Cardiac Outcomes
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose toxicity in β-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Brunner, Y.; Schvartz, D.; Priego-Capote, F.; Couté, Y.; Sanchez, J.C. Glucotoxicity and pancreatic proteomics. J. Proteom. 2009, 71, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, A.; De Filippis, E.A. Chronic hyperglycemia and nitric oxide bioavailability play a pivotal role in pro-atherogenic vascular modifications. Genes Nutr. 2007, 2, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Andreea, S.I.; Marieta, C.; Anca, D. AGEs and glucose levels modulate type I and III procollagen mRNA synthesis in dermal fibroblasts cells culture. Exp. Diabetes Res. 2008, 2008, 473603. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.G. Lipotoxicity: What is the fate of fatty acids? J. Lipid Res. 2008, 49, 1375–1376. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H. Lipid overload and overflow: Metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 2003, 14, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Guéant, J.L.; Elakoum, R.; Ziegler, O.; Coelho, D.; Feigerlova, E.; Daval, J.L.; Guéant-Rodriguez, R.M. Nutritional models of foetal programming and nutrigenomic and epigenomic dysregulations of fatty acid metabolism in the liver and heart. Pflugers Arch. Eur. J. Physiol. 2014, 466, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Laybutt, D.R.; Kaneto, H.; Bonner-Weir, S.; Sharma, A. β-cell adaptation and decompensation during the progression of diabetes. Diabetes 2001, 50 (Suppl. 1). [Google Scholar] [CrossRef]
- Prentki, M.; Joly, E.; El-Assaad, W.; Roduit, R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: Role in β-cell adaptation and failure in the etiology of diabetes. Diabetes 2002, 51 (Suppl. 3), S405–S413. [Google Scholar] [CrossRef] [PubMed]
- Véret, J.; Bellini, L.; Giussani, P.; Ng, C.; Magnan, C.; Le Stunff, H. Roles of sphingolipid metabolism in pancreatic β cell dysfunction induced by lipotoxicity. J. Clin. Med. 2014, 3, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Stanley, W.C. Too much or not enough of a good thing? Cardiac glucolipotoxicity versus lipoprotection. J. Mol. Cell. Cardiol. 2011, 50, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mdaki, K.S.; Larsen, T.D.; Wachal, A.L.; Schimelpfenig, M.D.; Weaver, L.J.; Dooyema, S.D.R.; Louwagie, E.J.; Baack, M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. AJP Heart Circ. Physiol. 2016, 310, H681–H692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, L.A.; Uthlaut, A.B.; Long, N.M.; Zhang, L.; Ma, Y.; Smith, D.T.; Nathanielsz, P.W.; Ford, S.P. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod. Biol. Endocrinol. 2010, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Mercola, M.; Ruiz-Lozano, P.; Schneider, M.D. Cardiac muscle regeneration: Lessons from development. Genes Dev. 2011, 25, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Reinecke, H.; Pabon, L.M. Regeneration gaps. Observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006, 47, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Morez, C.; Noseda, M.; Paiva, M.A.; Belian, E.; Schneider, M.D.; Stevens, M.M. Enhanced efficiency of genetic programming toward cardiomyocyte creation through topographical cues. Biomaterials 2015, 70, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Lie, S.; Hui, M.; McMillen, I.C.; Muhlhausler, B.S.; Posterino, G.S.; Dunn, S.L.; Wang, K.C.; Botting, K.J.; Morrison, J.L. Exposure to rosiglitazone, a PPAR-γ agonist, in late gestation reduces the abundance of factors regulating cardiac metabolism and cardiomyocyte size in the sheep fetus. AJP Regul. Integr. Comp. Physiol. 2014, 308, R627–R635. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.H.; Boyn, A.M.; Kumarasamy, V.; Hsieh, A.; Head, S.I.; Lumbers, E.R. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat. Rec. 2003, 274, 952–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonker, S.S.; Zhang, L.; Louey, S.; Giraud, G.D.; Thornburg, K.L.; Faber, J.J. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J. Appl. Physiol. 2007, 102, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.A.; Matkovich, S.J. Cardiomyocytes structure, function and associated pathologies. Int. J. Biochem. Cell Biol. 2005, 37, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.J.; Heymann, M.A.; Rudolph, A.M. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am. J. Physiol. 1980, 238, H399–H405. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Jaswal, J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Sizonenko, S.V.; Bassett, N.S. The transition from fetus to neonate—An endocrine perspective. Acta Paediatr. 1999, 88, 7–11. [Google Scholar] [CrossRef]
- Hay, W.W. Placental transport of nutrients to the fetus. Horm. Res. 1994, 42, 215–222. [Google Scholar] [PubMed]
- Lopaschuk, G.D.; Spafford, M.A.; Marsh, D.R. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am. J. Physiol. 1991, 261, H1698–H1705. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Cargnoni, A.; Ceconi, C. Anti-ischaemic effect of ivabradine. Pharmacol. Res. 2006, 53, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, B.; Thrush, A.; Quizi, J.; Antoun, G.; McIntosh, N.; Al-dirbashi, O.; Patti, M.-E.; Harper, M.-E. Undernutrition during pregnancy in mice leads to dysfunctional cardiac muscle respiration in adult offspring. Biosci. Rep. 2015, 35, e00200. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, P.; Young, M.E.; Alcorn, J.L.; Moravec, C.S.; Frazier, O.H.; Taegtmeyer, H. Metabolic gene expression in fetal and failing human heart. Circulation 2001, 104, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.N.; Rader, T.A.; Park, S.; Bastin, J.; McCune, S.A.; Kelly, D.P. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 1996, 94, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Pytel, G.; Schrepper, A.; Amorim, P.; Färber, G.; Shingu, Y.; Mohr, F.W.; Schwarzer, M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc. Res. 2010, 86, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.G.; Todor, A.V.; Silverman, N.; Goldstein, S.; Sabbah, H.N. Abnormal mitochondrial respiration in failed human myocardium. J. Mol. Cell. Cardiol. 2000, 32, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.; Goussev, A.; Lesch, M.; Goldstein, S.; Sabbah, H. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J. Mol. Cell. Cardiol. 1998, 30, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Kypson, A.P.; Rodriguez, E.; Anderson, C.A.; Lehr, E.J.; Neufer, P.D. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 2009, 54, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Sena, S.; Theobald, H.; Sheng, X.; Wright, J.J.; Xia, X.H.; Aziz, S.; Johnson, J.I.; Bugger, H.; Zaha, V.G.; et al. Mitochondrial energetics in the heart in obesity-related diabetes: Direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 2007, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.-E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Grimsgaard, S.; Bønaa, K.H.; Jacobsen, B.K.; Bjerve, K.S. Plasma saturated and linoleic fatty acids are independently associated with blood pressure. Hypertension 1999, 34, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Tamaya-Mori, N.; Uemura, K.; Iguchi, A. Gender differences in the dietary lard-induced increase in blood pressure in rats. Hypertension 2002, 39, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.M.; Robillard, J.H.; Grose, M.; Lebel, P.F. Arachidonic acid does not share the antihypertensive properties of linoleic acid and fish oilomega-3 fatty acids in a model of angiotensin II-induced hypertension in the rat. Clin. Investig. Med. 1991, 14, 518–524. [Google Scholar]
- Sellmayer, A.; Danesch, U.; Weber, P.C. Effects of different polyunsaturated fatty acids on growth-related early gene expression and cell growth. Lipids 1996, 31 (Suppl. 374), S37. [Google Scholar] [CrossRef] [PubMed]
- Földes, G.; Vajda, S.; Lakó-Futó, Z.; Sármán, B.; Skoumal, R.; Ilves, M.; de Châtel, R.; Karádi, I.; Tóth, M.; Ruskoaho, H.; et al. Distinct modulation of angiotensin II-induced early left ventricular hypertrophic gene programming by dietary fat type. J. Lipid Res. 2006, 47, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes-Antrás, J.; Picatoste, B.; Ramírez, E.; Egido, J.; Tuñón, J.; Lorenzo, Ó. Targeting metabolic disturbance in the diabetic heart. Cardiovasc. Diabetol. 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid metabolism and toxicity in the heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.G. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim. Biophys. Acta 2011, 1813, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Bioenergetic origins of complexity and disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2015, 594, 2061–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grynberg, A.; Demaison, L. Fatty acid oxidation in the heart. J. Cardiovasc. Pharmacol. 1996, 28 (Suppl. 1), 11–17. [Google Scholar]
- Makinde, A.O.; Kantor, P.F.; Lopaschuk, G.D. Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol. Cell. Biochem. 1998, 188, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. AJP Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70 (Suppl. 3), 560S–569S. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Overturf, M.L. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension 1988, 11, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, Y.; Kanno, Y.; Takeyama, D.; Ishide, N.; Maruyama, Y.; Takahashi, T.; Ido, T.; Takishima, T. Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. Circulation 1990, 81, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Zahabi, A.; Deschepper, C.F. Long-chain fatty acids modify hypertrophic responses of cultured primary neonatal cardiomyocytes. J. Lipid Res. 2001, 42, 1325–1330. [Google Scholar] [PubMed]
- Aguila, M.B.; Alberto Mandarim-de-Lacerda, C.A. Blood pressure, ventricular volume and number of cardiomyocyte nuclei in rats fed for 12 months on diets differing in fat composition. Mech. Ageing Dev. 2001, 122, 77–88. [Google Scholar] [CrossRef]
- Carroll, J.F.; Braden, D.S.; Cockrell, K.; Mizelle, H.L. Obese hypertensive rabbits develop concentric and eccentric hypertrophy and diastolic filling abnormalities. Am. J. Hypertens. 1997, 10, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.C.; Sohal, R.S.; Sun, S.C.; Burch, G.E.; Colcolough, H.L. Lipid cardiomyopathy of the hypertrophied heart of goldthioglucose obese mice. J. Pathol. 1969, 97, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.M.; Henegar, J.R.; Brands, M.W.; Henegar, L.K.; Hall, J.E. Cardiovascular and renal responses to a high-fat diet in Osborne-Mendel rats. AJP Regul. Integr. Comp. Physiol. 2001, 281, R547–R552. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.; Lind, L.; Vessby, B.; Andrén, B.; Aro, A.; Lithell, H. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation 2001, 103, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Kim, J.K. New insights into insulin resistance in the diabetic heart. Trends Endocrinol. Metab. 2011, 22, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, D.S. Heart failure: The frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003, 26, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Taveras, E.M.; Gillman, M.W.; Oken, E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am. J. Hypertens. 2009, 22, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T.; Toko, H.; Okada, S.; Ikeda, H.; Yasuda, N.; Tateno, K.; Moriya, J.; Yokoyama, M.; Nojima, A.; et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J. Clin. Investig. 2010, 120, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, Y.R.; Finck, B.N.; Kim, H.J.; Higashimori, T.; Hong, E.G.; Lee, M.K.; Danton, C.; Deshmukh, S.; Cline, G.W.; et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-α causes insulin resistance in heart and liver. Diabetes 2005, 54, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; O’Shea, K.M.; Ramasamy, R. Insulin resistance: Metabolic mechanisms and consequences in the heart. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, L.; Hernández-García, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregón, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Franco Mdo, C.; Dantas, A.P.; Akamine, E.H.; Kawamoto, E.M.; Fortes, Z.B.; Scavone, C.; Tostes, R.C.; Carvalho, M.H.; Nigro, D. Enhanced oxidative stress as a potential mechanism underlying the programming of hypertension in utero. J. Cardiovasc. Pharmacol. 2002, 40, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, E.F.; Donner, D.G. Myocardial insulin resistance: An overview of its causes, effects, and potential therapy. In Insulin Resistance; Arora, S., Ed.; InTechOpen: London, UK, 2012; pp. 189–226. [Google Scholar]
- Stahl, A.; Gimeno, R.E.; Tartaglia, L.A.; Lodish, H.F. Fatty acid transport proteins: A current view of a growing family. Trends Endocrinol. Metab. 2001, 12, 266–273. [Google Scholar] [CrossRef]
- Park, S.H.; Gammon, S.R.; Knippers, J.D.; Paulsen, S.R.; Rubink, D.S.; Winder, W.W. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J. Appl. Physiol. 2002, 92, 2475–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñeiro, R.; Iglesias, M.J.; Gallego, R.; Raghay, K.; Eiras, S.; Rubio, J.; Diéguez, C.; Gualillo, O.; González-Juanatey, J.R.; Lago, F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005, 579, 5163–5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopaschuk, G.D.; Gamble, J. Acetyl-CoA carboxylase: An important regulator of fatty acid oxidation in the heart. Can. J. Physiol. Pharmacol. 1994, 72, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Huss, J.M.; Kelly, D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 2000, 20, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Sugden, M.C.; Holness, M.J. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch. Physiol. Biochem. 2006, 112, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.W.; Brooks, D.A.; Thornburg, K.L.; Morrison, J.L. Activation of IGF-2R stimulates cardiomyocyte hypertrophy in the late gestation sheep fetus. J. Physiol. 2012, 590, 5425–5437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikimi, T.; Maeda, N.; Matsuoka, H. The role of natriuretic peptides in cardioprotection. Cardiovasc. Res. 2006, 69, 318–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, D.P.; Strauss, A.W. Inherited cardiomyopathies. N. Engl. J. Med. 1994, 330, 913–919. [Google Scholar] [PubMed]
- Brookheart, R.T.; Michel, C.I.; Schaffer, J.E. As a matter of fat. Cell Metab. 2009, 10, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. AJP Heart Circ. Physiol. 2006, 291, H1489–H1506. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Chinali, M.; Lee, E.T.; Galloway, J.M.; Barac, A.; Panza, J.A.; Howard, B.V. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J. Hypertens. 2010, 28, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, R.; Oudit, G.Y.; Wang, X.; Zhang, L.; Ussher, J.R.; Lopaschuk, G.D.; Kassiri, Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. AJP Heart Circ. Physiol. 2009, 297, H2096–H2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Ohga, Y.; Tabayashi, N.; Kobayashi, S.; Sakata, S.; Misawa, H.; Tsuji, T.; Kohzuki, H.; Suga, H.; Taniguchi, S.; et al. Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats. AJP Heart Circ. Physiol. 2002, 282, H138–H148. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.G.; Parikh, P.; Shannon, R.P. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ. Res. 2006, 98, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Pulinilkunnil, T.; Kienesberger, P.C.; Nagendran, J.; Sharma, N.; Young, M.E.; Dyck, J.R.B. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int. J. Obes. 2014, 38, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pulinilkunnil, T.; Kienesberger, P.C.; Nagendran, J.; Waller, T.J.; Young, M.E.; Kershaw, E.E.; Korbutt, G.; Haemmerle, G.; Zechner, R.; Dyck, J.R.B. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 2013, 62, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Lv, L.; Li, H.; Yu, D.M. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc. Diabetol. 2012, 11, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakasaki, H.; Koya, D.; Schoen, F.J.; Jirousek, M.R.; Ways, D.K.; Hoit, B.D.; Walsh, R.A.; King, G.L. Targeted overexpression of protein kinase C 2 isoform in myocardium causes cardiomyopathy. Proc. Natl. Acad. Sci. USA 1997, 94, 9320–9325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, J.; Mazumder, P.K.; Hu, P.; Chakrabarti, G.; Roberts, M.W.; Ui, J.Y.; Cooksey, R.C.; Litwin, S.E.; Abel, E.D. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005, 146, 5341–5349. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; An, D.; Pulinilkunnil, T.; Qi, D.; Lau, H.C.S.; Abrahani, A.; Innis, S.M.; Rodrigues, B. Role of dietary fatty acids and acute hyperglycemia in modulating cardiac cell death. Nutrition 2004, 20, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.C.; Bartlett, J.J.; Perez, L.J.; Brunt, K.R.; Legare, J.F.; Hassan, A.; Kienesberger, P.C.; Pulinilkunnil, T. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. Biochim. Biophys. Acta 2016, 1861, 1893–1910. [Google Scholar] [CrossRef] [PubMed]
- Van De Weijer, T.; Schrauwen-Hinderling, V.B.; Schrauwen, P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc. Res. 2011, 92, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Yoon, K.H. Glucolipotoxicity in pancreatic β-cells. Diabetes Metab. J. 2011, 35, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Y.; Lloyd-Jones, D.M.; Khan, S.S. Association of body mass index with mortality in cardiovascular disease: New insights into the obesity paradox from multiple perspectives. Trends Cardiovasc. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Brouwer, I.A.; Geleijnse, J.M.; Hornstra, G. Saturated fat consumption and risk of coronary heart disease and ischemic stroke: A science update. Ann. Nutr. Metab. 2017, 70, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics-2015 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2015, 131, e29–e322. [Google Scholar] [PubMed]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.A.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Duda, M.K.; O’Shea, K.M.; Stanley, W.C. Omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: Mechanisms and clinical potential. Cardiovasc. Res. 2009, 84, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E.; Louw, J. Islet cell response to high fat programming in neonate, weanling and adolescent Wistar rats. JOP 2014, 15, 228–236. [Google Scholar] [PubMed]
- Gittes, G.K. Developmental biology of the pancreas: A comprehensive review. Dev. Biol. 2009, 326, 4–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miralles, F. TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J. Cell Biol. 1998, 143, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Dyntar, D.; Eppenberger-Eberhardt, M.; Maedler, K.; Pruschy, M.; Eppenberger, H.M.; Spinas, G.A.; Donath, M.Y. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes 2001, 50, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 2001, 276, 14890–14895. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Leri, A.; Cesselli, D.; Limana, F.; Safai, B.; Nadal-Ginard, B.; Anversa, P.; Kajstura, J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 2001, 50, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Lee, E.Y.; Han, S.J.; Kim, S.H.; Lee, B.W.; Ahn, C.W.; Cha, B.S.; Lee, H.C. Dual pathways of p53 mediated glucolipotoxicity-induced apoptosis of rat cardiomyoblast cell: Activation of p53 pro-apoptosis and inhibition of Nrf2-NQO1 anti-apoptosis. Metabolism 2012, 61, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lee, J.W.; Suh, Y.H.; Lee, H.J.; Lee, S.H.; Oh, Y.K.; Gao, B.; Jung, M.H. AICAR potentiates ROS production induced by chronic high glucose: Roles of AMPK in pancreatic β-cell apoptosis. Cell. Signal. 2007, 19, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, W.; Wang, G.; Guo, L.; Jiang, Y.; James Kang, Y. Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes 2002, 51, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S. Mitochondrial uncoupling: A key contributor to reduced cardiac efficiency in diabetes. Physiology 2006, 21, 250–258. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, A.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lo Iacono, O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunakaran, U.; Kim, H.J.; Kim, J.Y.; Lee, I.K. Guards and culprits in the endoplasmic reticulum: Glucolipotoxicity and β-cell failure in type II diabetes. Exp. Diabetes Res. 2012, 2012, 639762. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Lippai, M.; Low, P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. BioMed Res. Int. 2014, 2014, 832704. [Google Scholar] [CrossRef] [PubMed]
- Casas, S.; Gomis, R.; Gribble, F.M.; Altirriba, J.; Knuutila, S.; Novials, A. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes 2007, 56, 2284–2294. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, O.; Minamino, T.; Okada, K.I.; Shintani, Y.; Takashima, S.; Kato, H.; Liao, Y.; Okazaki, H.; Asai, M.; Hirata, A.; et al. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem. Biophys. Res. Commun. 2006, 340, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diab. Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Montezano, A.C.; Hebert, R.L.; Gray, S.P.; Di Marco, E.; Jha, J.C.; Cooper, M.E.; Jandeleit-Dahm, K.; Schiffrin, E.L.; Wilkinson-Berka, J.L.; et al. Oxidative stress, nox isoforms and complications of diabetes-potential targets for novel therapies. J. Cardiovasc. Transl. Res. 2012, 5, 509–518. [Google Scholar] [CrossRef] [PubMed]
| Fetal Heart | Developed Heart | |
|---|---|---|
| Main fuel source | Glucose | Fatty acids |
| Glucose transport | GLUT1 | GLUT4 |
| Insulin dependency | Insulin-independent | Insulin-dependent |
| Meeting energy demand | Anaerobic glycolysis | Fatty acid β-oxidation |
| Cardiac fatty acid uptake | FAT/CD36; FATP1 | FAT/CD36; FATP1 |
| Cardiac insulin resistance: | ||
| glucose oxidation | - | Decreased |
| Cardiac insulin resistance: | ||
| fatty acid oxidation | - | Normal or increased |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerf, M.E. Cardiac Glucolipotoxicity and Cardiovascular Outcomes. Medicina 2018, 54, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050070
Cerf ME. Cardiac Glucolipotoxicity and Cardiovascular Outcomes. Medicina. 2018; 54(5):70. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050070
Chicago/Turabian StyleCerf, Marlon E. 2018. "Cardiac Glucolipotoxicity and Cardiovascular Outcomes" Medicina 54, no. 5: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050070





