Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analyses
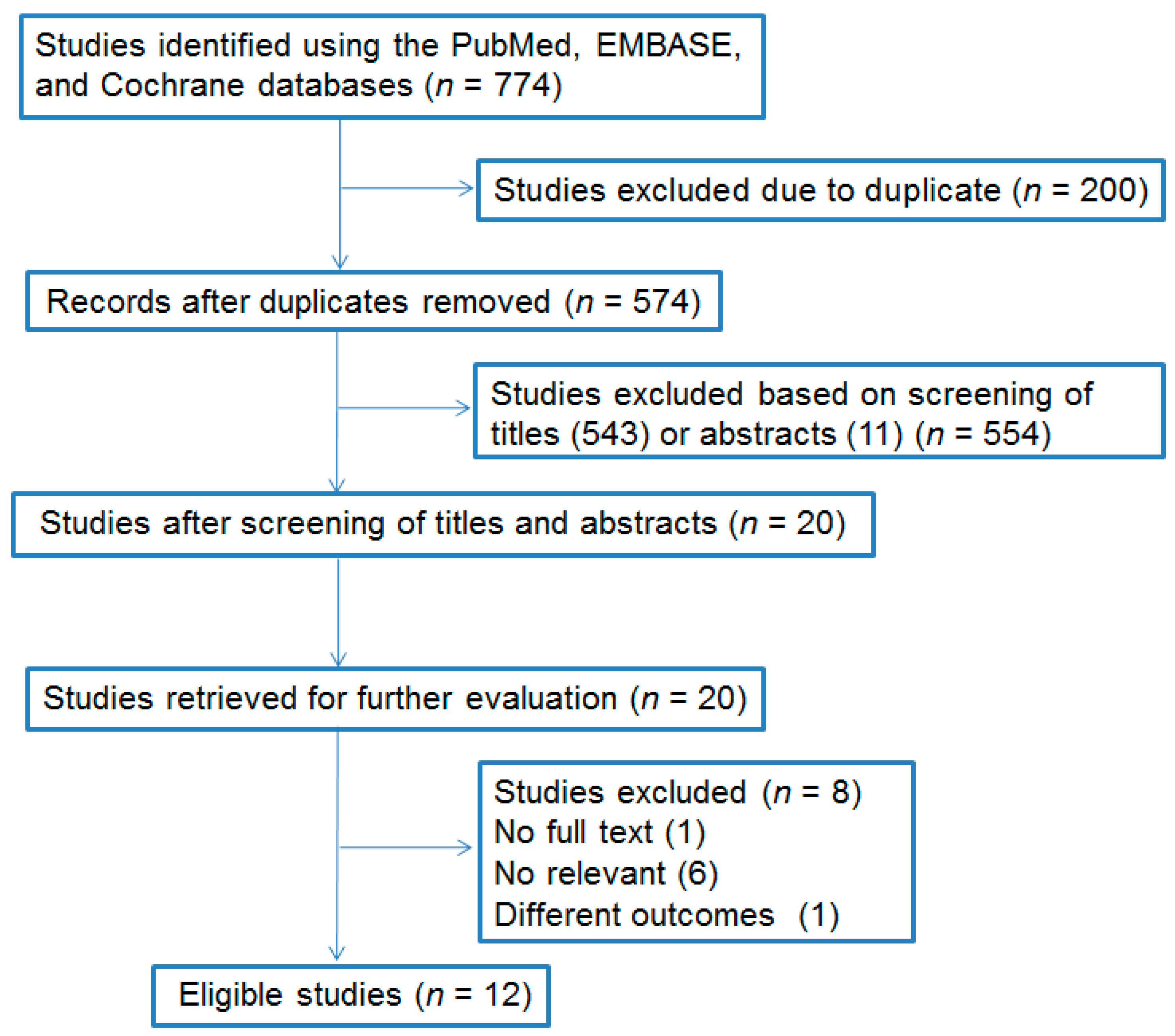
3. Results
3.1. Characteristics of the Included Studies
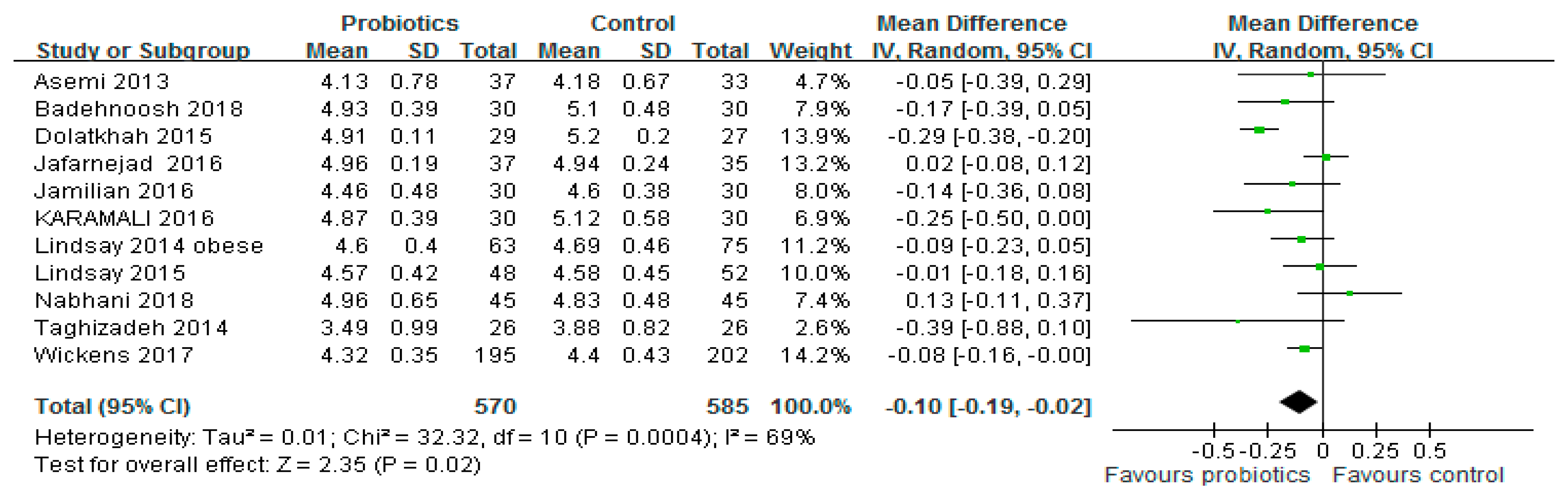
3.2. FBG Level
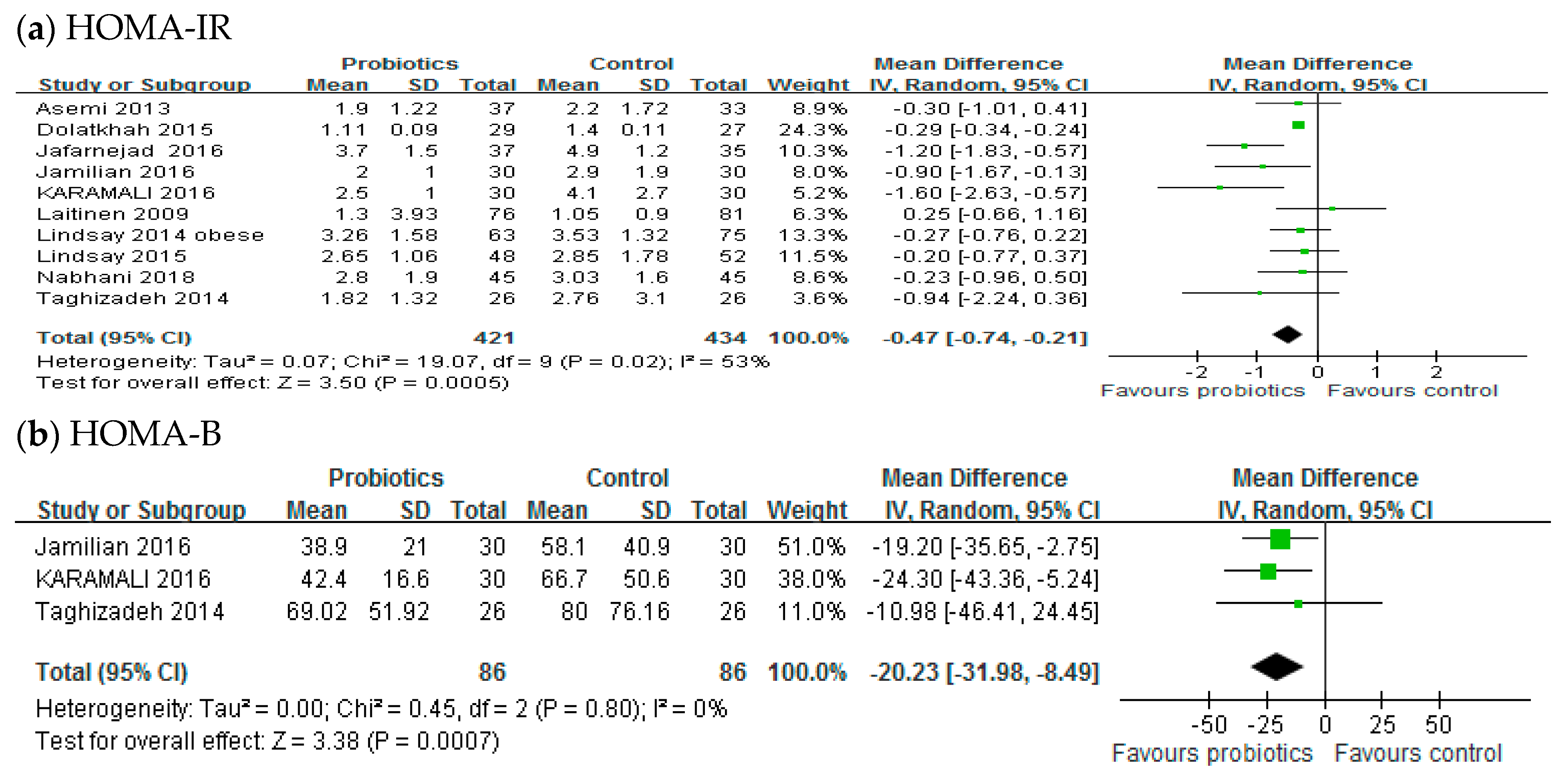
3.3. Insulin Resistance
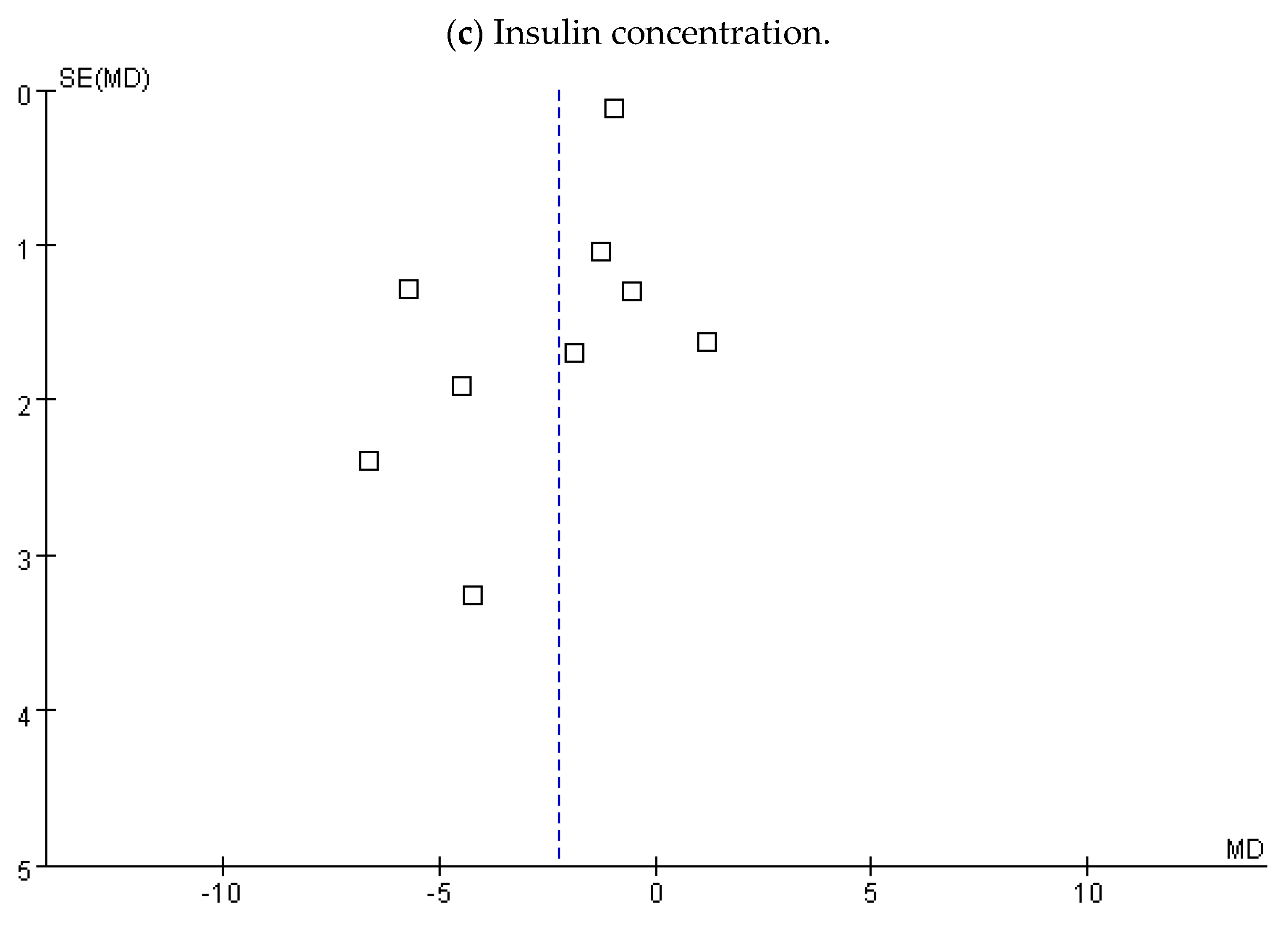
3.4. Insulin Concentration
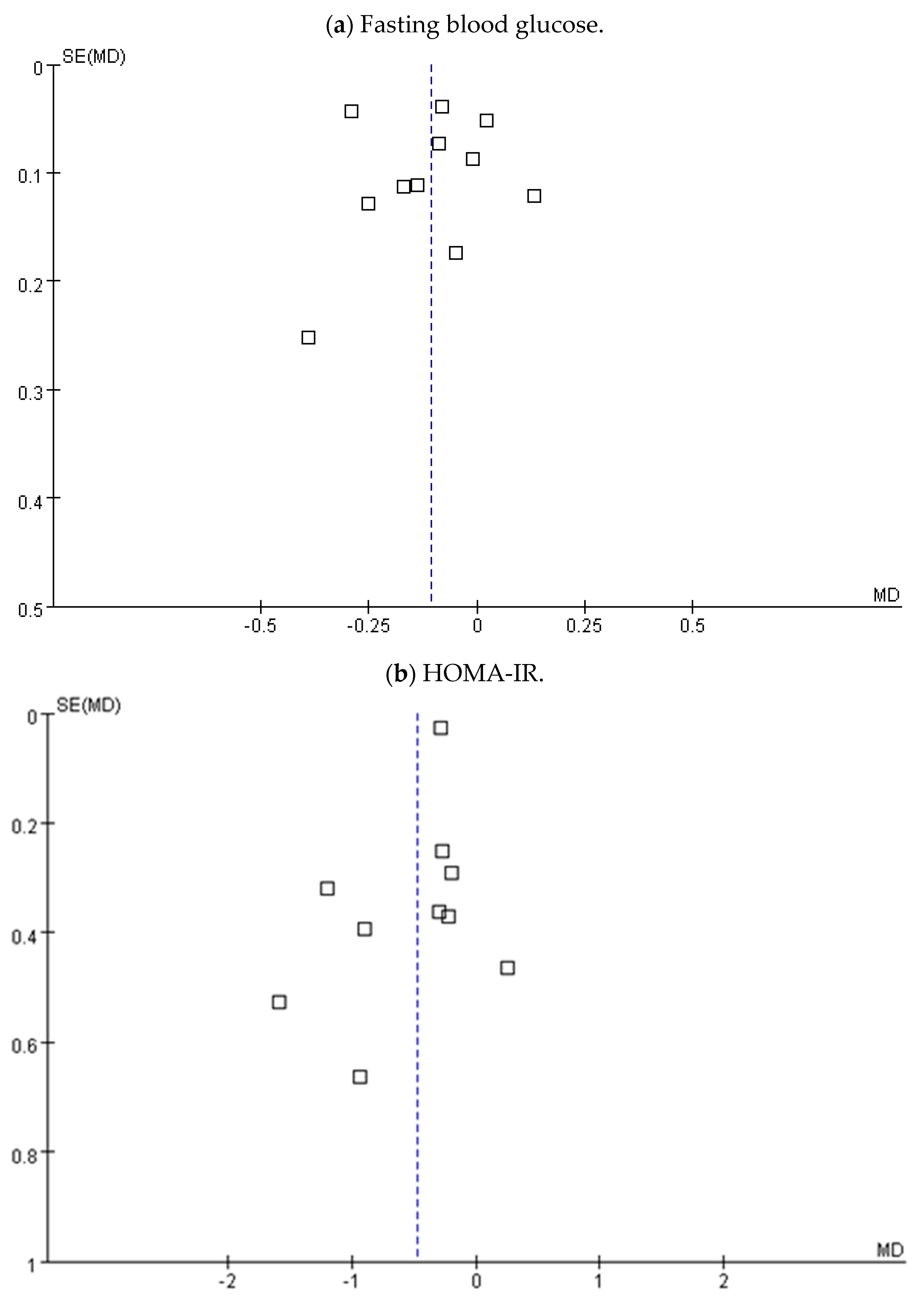
3.5. Subgroup Analysis, Sensitivity, and Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hadar, E.; Hod, M. Gestational diabetes and pregnancy outcome: Do we need an update on diagnostic criteria? Nutr. Metab. Cardiovasc. Dis. 2009, 19, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.L.; Dekker Nitert, M.; Conwell, L.S.; Callaway, L.K. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2014, 2, CD009951. [Google Scholar] [CrossRef] [PubMed]
- Kc, K.; Shakya, S.; Zhang, H. Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 14–20. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Xiong, G.; Bentley-Lewis, R. A systematic review of metabolite profiling in gestational diabetes mellitus. Diabetologia 2014, 57, 2453–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Hoeft, B.; Freerksen, N.; Fischer, B.; Roehrig, S.; Yamamoto, S.; Maul, H. Neonatal complications and risk factors among women with gestational diabetes mellitus. Acta Obstet. Gyn. Scand. 2011, 90, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Rimar, Z.; Milas, V.; Medimurec, M.; Mesić, I. Respiratory distress syndrome in newborns of gestational age of over 32 weeks. Coll. Antropol. 2014, 38, 621–626. [Google Scholar] [PubMed]
- Cheung, N.W. The management of gestational diabetes. Vasc. Health Risk Manag. 2009, 5, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Retnakaran, R.; Booth, G.L. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008, 31, 1668–1669. [Google Scholar] [CrossRef] [PubMed]
- Vohr, B.R.; Boney, C.M. Gestational diabetes: The forerunner for the development of maternal and childhood obesity and metabolic syndrome? J. Matern. Fetal Med. 2008, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Gilinsky, A.S.; Kirk, A.F.; Hughes, A.R.; Lindsay, R.S. Lifestyle interventions for type 2 diabetes prevention in women with prior gestational diabetes: A systematic review and meta-analysis of behavioural, anthropometric and metabolic outcomes. Prev. Med. Rep. 2015, 2, 448–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, R.X. Gestational diabetes mellitus: A review from 2004. Curr. Diabetes Rev. 2005, 1, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. Impact of metformin on reproductive tissues: An overview from gametogenesis to gestation. Ann. Transl. Med. 2014, 2, 55. [Google Scholar] [PubMed]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Zok, C. The importance of the human intestinal microbiota. Dtsch. Med. Wochenschr. 2014, 139, 1282–1283. [Google Scholar] [PubMed]
- Morelli, L. Yogurt, living cultures, and gut health. Am. J. Clin. Nutr. 2014, 99 (Suppl. 5), 1248S–1250S. [Google Scholar] [CrossRef] [PubMed]
- Moro-Garcia, M.A.; Alonso-Arias, R.; Baltadjieva, M.; Fernández Benítez, C.; Fernández Barrial, M.A.; Díaz Ruisánchez, E.; Alonso Santos, R.; Alvarez Sánchez, M.; Saavedra Miján, J.; López-Larrea, C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 2013, 35, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, X.M.; Zhang, Q.X.; Shen, Z.; Tian, F.W.; Zhang, H.; Sun, Z.H.; Zhang, H.P.; Chen, W. Influence of consumption of probiotics on the plasma lipid profile: A meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Alokail, M.S.; Sabico, S.; Al-Saleh, Y.; Al-Daghri, N.M.; Alkharfy, K.M.; Vanhoutte, P.M.; McTernan, P.G. Effects of probiotics in patients with diabetes mellitus type 2: Study protocol for a randomized, double-blind, placebo-controlled trial. Trials 2013, 14, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitert, M.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Wilkinson, S.; Lingwood, B.; Tobin, J.M.; McSweeney, C.; O’Rourke, P.; McIntyre, H.D.; et al. SPRING: An RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy Child. 2013, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Bordalo Tonucci, L.; Dos Santos, K.M.; De Luces Fortes Ferreira, C.L. Gut microbiota and probiotics: Focus on diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2017, 57, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Kootte, R.S.; Vrieze, A.; Holleman, F.; allinga-Thie, G.M.; Zoetendal, E.G.; de Vos, W.M.; Groen, A.K.; Hoekstra, J.B.; Stroes, E.S.; Nieuwdorp, M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes. Metab. 2012, 14, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Ozaki, M.; Tamura, A.; Yamada, N.; Ishida, T.; Hosoda, M.; Hosono, A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2003, 67, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, E461. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Asemi, Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: A randomized controlled clinical trial. Hormones 2014, 13, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Naghibi Rad, M.; Rahimi Foroushani, A.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Kennelly, M.; Culliton, M.; Smith, T.; Maguire, O.C.; Shanahan, F.; Brennan, L.; McAuliffe, F.M. Probiotics in obese pregnancy do not reduce maternal fasting glucose: A double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am. J. Clin. Nutr. 2014, 99, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e1-11. [Google Scholar] [PubMed]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: A randomised controlled trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Bahmani, F.; Vahedpoor, Z.; Salmani, A.; Tajabadi-Ebrahimi, M.; Jafari, P.; Hashemi Dizaji, S.; Asemi, Z. Effects of Probiotic Supplementation on Metabolic Status in Pregnant Women: A Randomized, Double-blind, Placebo-Controlled Trial. Arch. Iran. Med. 2016, 19, 687–692. [Google Scholar] [PubMed]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern. Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; sghari-Jafarabadi, M.; Gargari, B.P. The effects of symbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Deeks, J.J.; Altman, D.G. Special topics in statistics. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Book Series; The Cochrane Collaboration: Chichester, UK, 2008; pp. 48–529. [Google Scholar]
- Laitinen, K.; Poussa, T.; Isolauri, E.; Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotic and dietary counselling contribute to glucose regulation during and after pregnancy: A randomized controlled trial. Br. J. Nutr. 2009, 101, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, F.M.; Colado Simão, A.N.; Morimoto, H.K.; Batisti Lozovoy, M.A.; Dichi, I.; Helena da Silva Miglioranza, L. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Aris, N.M.; Mahdy, Z.A.; Ahmad, S.; Naim, N.M.; Siraj, H.H.; Zakaria, S.Z. Gestational diabetes mellitus in primigravidae: A mild disease. Acta Med. 2011, 54, 21–24. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.Y.; Baik, S.H.; Woo, J.T.; Kwon, Y.J.; Daily, J.W.; Park, Y.M.; Yang, J.H.; Kim, S.H. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur. J. Clin. Nutr. 2016, 67, 196–201. [Google Scholar] [CrossRef] [PubMed]



| Study | Design | Intervention/Control (Sample Size) | Age | Duration (Weeks) | Probiotic | Probiotic Source | Dose (CFU) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Asemi et al. [29] | SB | Probiotic yogurt/Conventional yogurt (37/33) | 18–30 | 9 | L. acidophilus, L. bulgaricus, S. thermophiles, B. animals | Y | 1 × 107 | FBG HOMA-IR Insulin |
| Laitinen et al. [41] | DB | Probiotic/placebo (66/70) | 25–35 | 20 | L. rhamnosus, B. lactis | C | 1 × 1010 | HOMA-IR Insulin |
| Lindsay et al. [30] | DB | Probiotic/placebo (63/75) | 26–36 OB | 4 | L. salivarius | C | 1 × 109 | FBG HOMA-IR Insulin |
| Karamali et al. [31] | DB | Probiotic/placebo (30/30) | 18–40 | 6 | L. acidophilus, L. casei, B. bifidum | C | 6 × 109 | FBG HOMA-IR Insulin HOMA-B |
| Dolatkhah et al. [27] | DB | Probiotic/placebo (29/27) | 18–45 | 8 | L. acidophilus, Bifidobacterium, S. thermophiles, L. bulgaricus | C | 4 × 109 | FBG HOMA-IR Insulin |
| Jafarnejad et al. [32] | DB | Probiotic/placebo (37/35) | 32.4 ± 3.1, 31.9 ± 4.0 | 8 | B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. Bulgaricus | C | 15 × 109 | FBG HOMA-IR Insulin |
| Lindsay et al. [33] | DB | Probiotic/placebo (48/52) | >18 | 6 | L. salivarius | C | 1 × 109 | FBG HOMA-IR Insulin |
| Taghizadeh et al. [28] | TB | Synbiotic/placebo (26/26) | 18–35 | 9 | L. sporogenes | C | 18 × 107 | FBG HOMA-IR Insulin HOMA-B |
| Wickens et al. [34] | DB | Probiotic/placebo (195/202) | >16 | >12 | L. rbamnosus HN001 | C | 6 × 109 | FBG |
| Jamilian et al. [35] | DB | Probiotic/placebo (30/30) | 18–37 | 12 | L. acidophilus, L. casei, B. bifidum | C | 6 × 109 | FBG HOMA-IR Insulin HOMA-B |
| Badehnoosh et al. [36] | DB | Probiotic/placebo (30/30) | 18–40 | 6 | L. acidophilus, L. casei, B. bifidum | C | 6 × 109 | FBG |
| Nabhani et al. [37] | DB | Symbiotic/placebo (45/45) | 18–40 | 6 | L. acidophilus, L. plantarum, L. fermentum, L. gasseri | C | 2.5 × 1010 7.5 × 109 3.5 × 109 1 × 1010 | FBG HOMA-IR |
| Reference | Adequate Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data Addressed | Free of Selective Reporting | Free of Other Bias * |
|---|---|---|---|---|---|---|
| Asemi et al. [29] | Yes | Unclear | Yes | Yes | Yes | Yes |
| Laitinen et al. [41] | Yes | Yes | Yes | Yes | Yes | Yes |
| Lindsay et al. [30] | Yes | Yes | Yes | Yes | Yes | Unclear |
| Karamali et al. [31] | Yes | Yes | Yes | Yes | Yes | Unclear |
| Dolatkhah et al. [27] | Yes | Yes | Yes | Yes | Yes | Unclear |
| Jafarnejad et al. [32] | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Lindsay et al. [33] | Yes | Yes | Yes | Yes | Yes | Unclear |
| Taghizadeh et al. [28] | Yes | Yes | Yes | Yes | Yes | Unclear |
| Wickens et al. [34] | Yes | Yes | Yes | Yes | Yes | Unclear |
| Jamilian et al. [35] | Yes | Yes | Yes | Unclear | Yes | Unclear |
| Badehnoosh et al. [36] | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Nabhani et al. [37] | Yes | Yes | Yes | Yes | Yes | Unclear |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-R.; Wu, T.-W.; Chao, Y.-C. Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials. Medicina 2018, 54, 77. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050077
Peng T-R, Wu T-W, Chao Y-C. Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials. Medicina. 2018; 54(5):77. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050077
Chicago/Turabian StylePeng, Tzu-Rong, Ta-Wei Wu, and You-Chen Chao. 2018. "Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials" Medicina 54, no. 5: 77. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050077





