Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis
Abstract
:1. Background and Objectives
2. Material and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Speckle Tracking Echocardiography
2.4. Statistical Analysis
3. Results
3.1. LV Geometry, Function and Deformation Analysis
3.2. RV Geometry, Function and Deformation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Nicola, L.; Minutolo, R. Worldwide growing epidemic of CKD: Fact or fiction? Kidney Int. 2016, 90, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hsu, C.; Li, Y.; Mishra, R.K.; Keane, M.; Rosas, S.E.; Dries, D.; Xie, D.; Chen, J.; He, J.; et al. Associations between Kidney Function and Subclinical Cardiac Abnormalities in CKD. J. Am. Soc. Nephrol. 2012, 23, 1725–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrakanas, T.A.; Charytan, D.M. Cardiovascular complications in chronic dialysis patients. Curr. Opin. Nephrol. Hypertens. 2016, 25, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, M.; Karumanchi, S.A.; Thadhani, R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation 2016, 133, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Al-Biltagi, M.; Tolba, O.A.; ElHafez, M.A.; Abo-Elezz, A.A.; El Kady, E.K.; Hazza, S.M. Oxidative stress and cardiac dysfunction in children with chronic renal failure on regular hemodialysis. Pediatr. Nephrol. 2016, 31, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Ahmad, A. Cardiorenal syndromes. World J. Cardiol. 2011, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ishii, H.; Takahashi, H.; Aoyama, T.; Morita, Y.; Kasuga, H.; Kimura, K.; Ito, Y.; Takahashi, R.; Toriyama, T.; et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Mangrum, J.; Lin, D.; Dimarco, J.; Lake, D.; Bolton, W.; Mangrum, A. Prognostic value of left ventricular systolic function in renal dialysis patients. Heart Rhythm. 2006, 3, 154. [Google Scholar] [CrossRef]
- Edwards, N.C.; Hirth, A.; Ferro, C.J.; Townend, J.N.; Steeds, R.P. Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: The precursor of uremic cardiomyopathy? J. Am. Soc. Echocardiogr. 2008, 21, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Blessberger, H.; Binder, T. Two dimensional speckle tracking echocardiography: Basic principles. Heart 2010, 96, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Erpenbeck, J.; Schneider, R.K.; Röhl, A.B.; Hein, M.; Brandenburg, V.M.; van Diepen, M.; Dekker, F.; Marx, N.; Floege, J.; et al. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J. Am. Soc. Nephrol. 2014, 25, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.Y.; Green, D.; Abidin, N.; Sinha, S.; Kalra, P.A. Cardiac imaging in patients with chronic kidney disease. Nat. Rev. Nephrol. 2015, 11, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Su, C.T.; Song, E.J.; Tsai, W.C.; Li, Y.H.; Tsai, L.M.; Chen, J.-H.; Sung, J.-M. The role of echocardiographic study in patients with chronic kidney disease. J. Formos. Med. Assoc. 2015, 114, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.D.; Dubin, R.F.; Deo, R.; Daruwalla, V.; Friedman, J.L.; Medina, C.; Beussink, L.; Freed, B.H.; Shah, S.J. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2016, 18, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, X.; Shen, L.J.; Wang, B.; Ma, M.M.; Yang, Y.; Zhao, B.W. Left ventricular myocardial function in hemodialysis and nondialysis uremia patients: A three-dimensional speckle-tracking echocardiography study. PLoS ONE 2014, 9, e100265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Su, C.T.; Huang, Y.Y.; Yang, C.S.; Huang, J.W.; Yang, M.T.; Chen, J.-H.; Tsai, W.-C. Left ventricular systolic strain in chronic kidney disease and hemodialysis patients. Am. J. Nephrol. 2011, 33, 84–90. [Google Scholar] [CrossRef] [PubMed]
- International Society of Nephrology. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 5–14. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Foppa, M.; Duncan, B.B.; Rohde, L.E. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc. Ultrasound 2005, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Kalra, P.R.; Kalra, P.A. Echocardiographic abnormalities in dialysis patients with normal ejection fraction. Nephrol. Dial. Transplant. 2012, 27, 4256–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segall, L.; Nistor, I.; Covic, A. Heart failure in patients with chronic kidney disease: A systematic integrative review. Biomed. Res. Int. 2014, 2014, 937398. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Benedetto, F.A.; Tripepi, G.; Mallamaci, F. Cardiac consequences of hypertension in hemodialysis patients. Semin. Dial. 2004, 17, 299–303. [Google Scholar] [CrossRef] [PubMed]
- De Bie, M.K.; Marsan, N.A.; Gaasbeek, A.; Bax, J.J.; Groeneveld, M.; Gabreels, B.A.; Delgado, V.; Rabelink, T.J.; Schalij, M.J.; Jukema, J.W. Left ventricular diastolic dysfunction in dialysis patients assessed by novel speckle tracking strain rate analysis: Prevalence and determinants. Int. J. Nephrol. 2012, 2012, 963504. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Benedetto, F.A.; Mallamaci, F.; Tripepi, R.; Malatino, L.; Zoccali, C. Left atrial volume in end-stage renal disease: A prospective cohort study. J. Hypertens. 2006, 24, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Benedetto, F.A.; Mallamaci, F.; Tripepi, G.; Giacone, G.; Cataliotti, A.; Seminara, G.; Stancanelli, B.; Malatino, L.S. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J. Am. Soc. Nephrol. 2004, 15, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Wanner, C. The challenge of sudden death in dialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barré, P.E. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J. Am. Soc. Nephrol. 1995, 5, 2024–2031. [Google Scholar] [PubMed]
- Hickson, L.J.; Negrotto, S.M.; Onuigbo, M.; Scott, C.G.; Rule, A.D.; Norby, S.M.; Albright, R.C.; Casey, E.T.; Dillon, J.J.; Pellikka, P.A.; et al. Echocardiography Criteria for Structural Heart Disease in Patients with End-Stage Renal Disease Initiating Hemodialysis. J. Am. Coll. Cardiol. 2016, 67, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Karavelioğlu, Y.; Özkurt, S.; Kalçik, M.; Karapinar, H.; Arisoy, A. Echocardiographic assessment of right ventricular functions in nondiabetic normotensive hemodialysis patients. Interv. Med. Appl. Sci. 2015, 7, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, L.P.; Roger, S.D.; Levin, A. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J. Am. Soc. Nephrol. 2004, 15, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- London, G. Pathophysiology of cardiovascular damage in the early renal population. Nephrol. Dial. Transplant. 2001, 16 (Suppl. 2), 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneni, F.; Gregori, M.; Ciavarella, G.M.; Sciarretta, S.; De Biase, L.; Marino, L.; Tocci, G.; Principe, F.; Domenici, A.; Luciani, R.; et al. Right ventricular dysfunction in patients with end-stage renal disease. Am. J. Nephrol. 2010, 32, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Gregori, M.; Ciavarella, G.M.; Sciarretta, S.; Palano, F.; Pignatelli, G.; Castello, L.; Domenici, A.; Punzo, G.; Tocci, G.; et al. Relation between right and left ventricular function in patients undergoing chronic dialysis. J. Cardiovasc. Med. 2013, 14, 289–295. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Control Group N = 32 | Haemodialysis Group N = 38 | p Value |
|---|---|---|---|
| Age, median (IQR) | 57.2 (56–59.75) | 58.5 (49.8–72.0) | 0.741 |
| Gender, n (male:female) | 12:20 | 19:19 | 0.340 |
| Height (m) | 1.68 (1.54–1.82) | 1.71 (1.57–1.85) | 0.761 |
| Weight (kg) | 77.46 (13.77) | 79.28 (19.71) | 0.543 |
| BSA (m2) | 1.89 (0.20) | 1.91 (0.25) | 0.671 |
| AH, n (%) | 5 (15.6) | 8 (21.1) | 0.395 |
| DM, n (%) | 4 (12.5) | 6 (15.8) | 0.484 |
| HD vintage, mean (SD), years | NA | 3.84 (0.69) | |
| HD vintage, n (%) | |||
| <1 year | 5 (13.2) | ||
| 1–5 years | 23 (60.5) | ||
| >5 years | 10 (26.3) | ||
| Secondary anaemia, n (%) | NA | 36 (94.7) | |
| Secondary hyperparathyroidism, n (%) | NA | 26 (68.4) | |
| Hyperuricaemia, n (%) | NA | 6 (15.8) | |
| ACE inhibitors/ARB, n (%) | 5 (15.6) | 22 (57.9) | <0.001 |
| Beta blockers, n (%) | 0 (0.0) | 23 (60.5) | <0.001 |
| Characteristic | Control Group N = 32 | Haemodialysis Group N = 38 | p Value |
|---|---|---|---|
| LVEDDi (mm/m2) | 25.31 (2.69) | 24.26 (2.56) | 0.110 |
| LVMM (g) | 163.33 (44.14) | 217.61 (67.29) | <0.001 |
| LVMMi (g/m2) | 84.21 (16.99) | 111.64 (27.99) | <0.001 |
| Simpson LVEF (%) | 66.81 (6.77) | 63.97 (5.94) | 0.072 |
| LV S′ septal (cm/s) | 9.00 (8–10) | 8.27 (7.02–9.52) | 0.004 |
| LV S′ lateral (cm/s) | 11.19 (2.75) | 9.83 (3.24) | 0.020 |
| LV S′ inferior (cm/s) | 10.00 (8.88–11.13) | 9.11 (7.11–11.11) | 0.012 |
| LV S′ anterior (cm/s) | 10.92 (1.93) | 9.11 (2.22) | <0.001 |
| LA volume index (mL/m2) | 26.12 (6.31) | 40.32 (4.09) | <0.001 |
| E/A ratio | 1.18 (0.21) | 0.95 (0.29) | <0.001 |
| E/e′ | 5.83 (1.14) | 9.94 (4.04) | <0.001 |
| Characteristic | Control Group N = 32 | Haemodialysis Group N = 38 | p Value |
|---|---|---|---|
| RA volume index (mL/m2) | 19.98 (5.29) | 25.85 (10.95) | 0.012 |
| PA diameter (mm) | 20.19 (2.71) | 23.71 (2.38) | 0.007 |
| mPAP (mmHg) | 18.97 (8.60) | 24.60 (8.96) | 0.019 |
| RVD1 (mm) | 32.37 (5.72) | 33.18 (3.99) | 0.490 |
| TAPSE (mm) | 26.67 (22.8–30.54) | 20.50 (15.44–25.57) | 0.001 |
| RV S′ (cm/s) | 15.57 (1.63) | 12.42 (3.04) | <0.001 |
| RV FAC (%) | 58.11 (7.49) | 55.67 (7.39) | 0.191 |
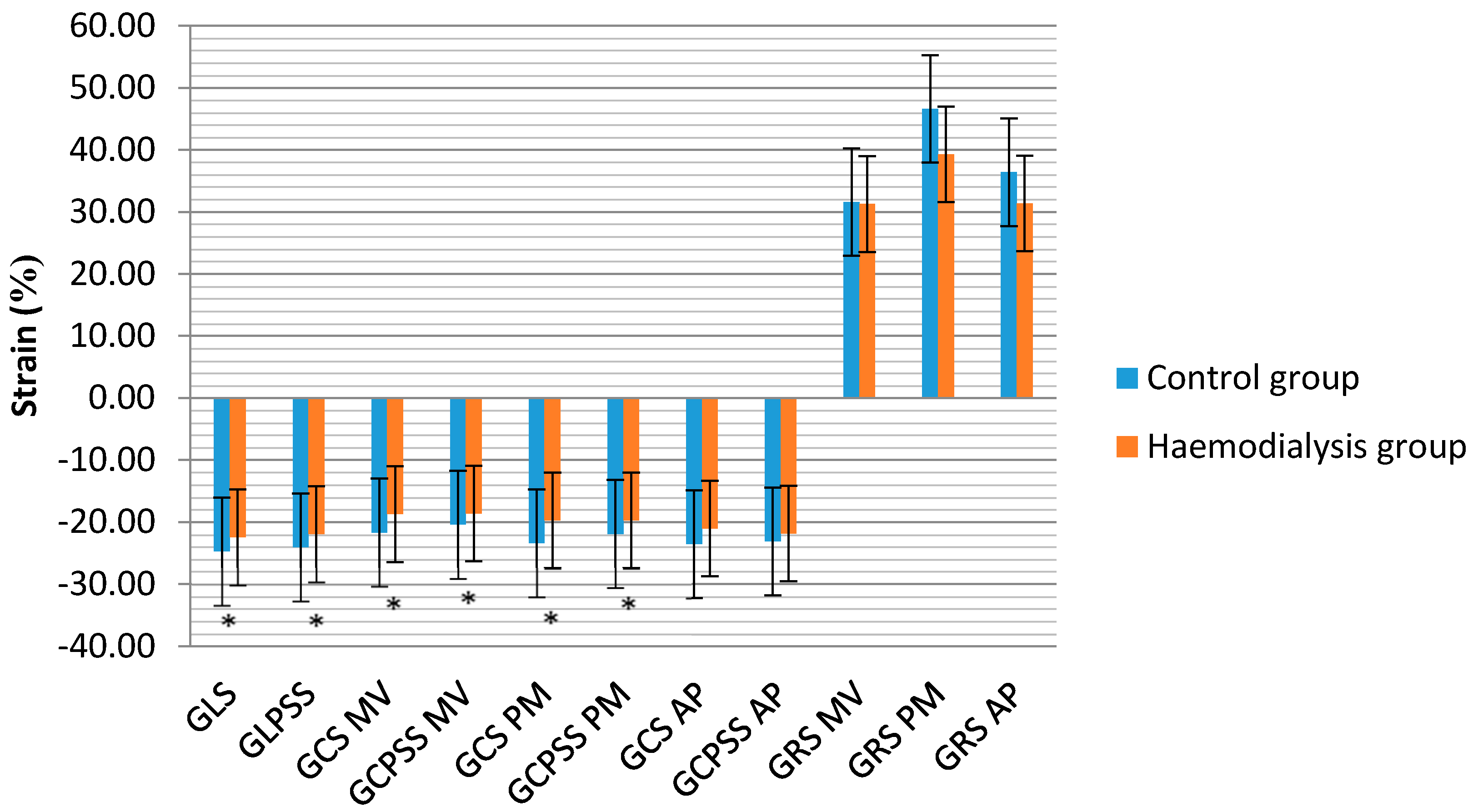
| RV GLS (%) | −25.45 (2.48) | −22.96 (3.04) | <0.001 |
| RV GLPSS (%) | −25.07 (−26.47–(−23.67)) | −24.65 (−25.95–(−23.35)) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamulėnaitė, E.; Žvirblytė, R.; Ereminienė, R.; Žiginskienė, E.; Ereminienė, E. Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis. Medicina 2018, 54, 87. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050087
Tamulėnaitė E, Žvirblytė R, Ereminienė R, Žiginskienė E, Ereminienė E. Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis. Medicina. 2018; 54(5):87. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050087
Chicago/Turabian StyleTamulėnaitė, Eglė, Rūta Žvirblytė, Rūta Ereminienė, Edita Žiginskienė, and Eglė Ereminienė. 2018. "Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis" Medicina 54, no. 5: 87. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050087




