Inflammation and Endotyping in Chronic Rhinosinusitis—A Paradigm Shift
Abstract
:1. An Introduction to Chronic Rhinosinusitis
2. The Role of the Immune System in the Upper Airways
2.1. CRS—A Microbiome in Dysbiosis?
2.2. The Role of the Mucociliary System
2.3. Innate Immunity and Epithelial Immunity
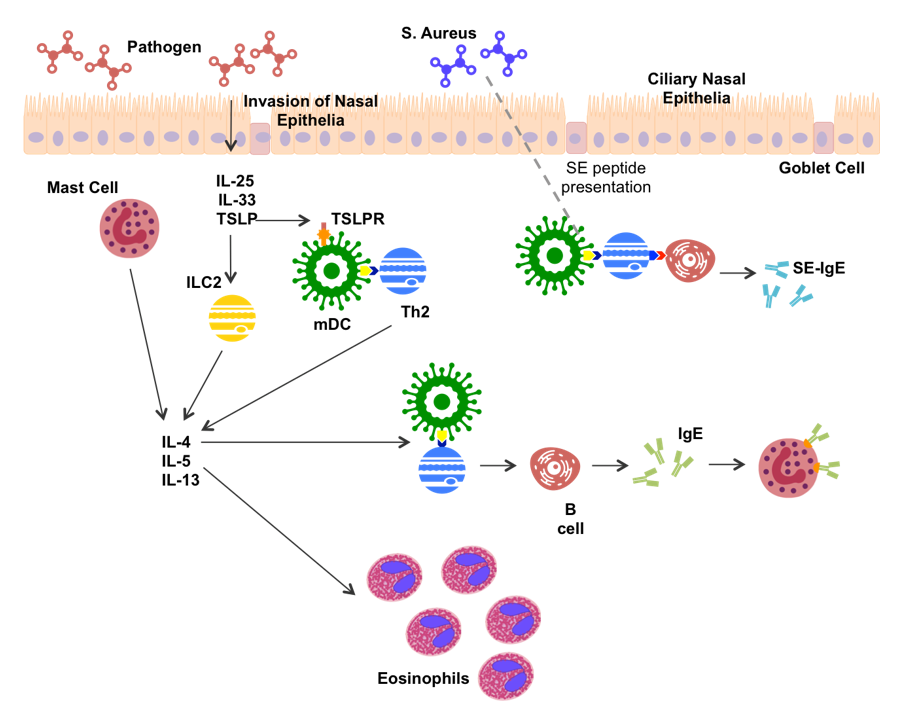
2.4. Recognition of Non-Self
3. CRS—A Chronic Inflammatory Disease
3.1. The Role of T-Effector Cells
3.2. The Geographical Conundrum
3.3. Type 2 Inflammation Is Well Characterized
3.4. Non-Type 2 Inflammation—A New Concept
4. The Emergence of Endotyping
4.1. Endotyping by Inflammatory Markers
- Neutrophilic inflammation characterized by pro-inflammatory cytokines IL-1β, IL-6, IL-8 and Myeloperoxidase
- Th17- or Th22- driven inflammation characterized by IL-17, IL-22
- Th1-driven inflammation characterized by IFN-γ
4.2. Endotyping by Clinical Features
4.3. Endotyping by Microbial Composition
4.4. Endotyping by Nasal Secretions
4.5. Endotyping-Still Under Development
5. Treatment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, N.; Velez, F.; Mahlis, E.; Medicis, J.; Messina, J.; Beckerman, R.; Gricar, J.; Beckerman, R.; Mahmoud, R.A. Economic Impact Associated with a Reduction in Surgical Eligibility Among Adult Patients with Chronic Rhinosinusitis—A Population Cost Offset Model. J. Allergy Clin. Immunol. 2018, 141, AB165. [Google Scholar] [CrossRef]
- Australian Health Survey: First Results, 2011–12; Survey; Australian Bureau of Statistics: Canberra, Australia, 2012.
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European Position Paper on Rhinosinusitis and Nasal Polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Crombruggen, K.; van Bruaene, N.; Holtappels, G.; Bachert, C. Chronic Sinusitis and Rhinitis: Clinical Terminology “Chronic Rhinosinusitis” Further Supported. Rhinology 2010, 48, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M.; Andes, D.; Neil, B.; Cheung, D.; Eisenberg, S.; Ganiats, T.G.; Gelzer, A.; Hamilos, D.; Haydon, R.C.; Hudgins, P.A. Clinical Practice Guideline: Adult sinusitis. Otolaryngol. Head Neck Surg. 2007, 137, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The Microbiome of the Middle Meatus in Healthy Adults. PLoS ONE 2014, 8, e85507. [Google Scholar] [CrossRef] [PubMed]
- Bordin, A.; Sidjabat, H.E.; Cottrell, K.; Cervin, A. Chronic Rhinosinusitis: A Microbiome in Dysbiosis and the Search for Alternative Treatment Options. Microbiol. Aust. 2016, 37, 149–152. [Google Scholar]
- Feazel, L.M.; Robertson, C.E.; Ramakrishnan, V.R.; Frank, D.N. Microbiome Complexity and Staphylococcus Aureus in Chronic Rhinosinusitis. Laryngoscope 2012, 122, 467–472. [Google Scholar] [CrossRef]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus Microbiome Diversity Depletion and Corynebacterium Tuberculostearicum Enrichment Mediates Rhinosinusitis. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Choi, E.B.; Hong, S.W.; Kim, D.K.; Jeon, S.G.; Kim, K.R.; Cho, S.H.; Gho, Y.S.; Jee, Y.K.; Kim, Y.K. Decreased Diversity of Nasal Microbiota and their Secreted Extracellular Vesicles in Patients with Chronic Rhinosinusitis Based an a Metagenomic Analysis. Allergy 2014, 69, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Keshavarzian, A.; Tobin, M.C.; Landay, A.L.; Schleimer, R.P. A Comprehensive Review of the Nasal Microbiome in Chronic Rhinosinusitis (CRS). Clin. Exp. Allergy 2016, 46, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Copeland, E.; Leonard, K.; Carney, R.; Kong, J.; Forer, M.; Naidoo, Y.; Oliver, B.G.G.; Seymour, J.R.; Woodcock, S.; Burke, C.M.; et al. Chronic Rhinosinusitis: Potential Role of Microbial Dysbiosis and Recommendations for Sampling Sites. Front. Cell Infect. Microbiol. 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.K.; Koeppel, A.F.; Hendley, J.O.; Turner, S.D.; Winther, B.; Sale, M.M. Characterization of the Nasopharyngeal Microbiota in Health and During Rhinovirus Challenge. Microbiome 2014, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Chalermwatanachai, T.; Zhang, N.; Holtappels, G.; Bachert, C. Association of Mucosal Organisms with Patterns of Inflammation in Chronic Rhinosinusitis. PLoS ONE 2015, 10, e0136068. [Google Scholar] [CrossRef] [PubMed]
- Van Zele, T.; Gevaert, P.; Watelet, J.B.; Claeys, G.; Holtappels, G.; Claeys, C.; van Cauwenberge, P.; Bachert, C. Staphylococcus Aureus colonization and ige antibody formation to enterotoxins is increased in nasal polyposis. J. Allergy Clin. Immunol. 2004, 114, 981–983. [Google Scholar] [PubMed]
- Schmidt, F.; Meyer, T.; Sundaramoorthy, N.; Michalik, S.; Surmann, K.; Depke, M.; Dhople, M.; Gesell Salazar, M.; Holtappels, G.; Zhang, N.; et al. Characterization of Human and Staphylococcus Aureus Proteins in Respiratory Mucosa By In Vivo- And Immunoproteomics. J. Proteomics 2017, 155, 31–39. [Google Scholar] [CrossRef]
- Schwartz, J.S.; Peres, A.G.; Endam, L.M.; Cousineau, B.; Madrenas, J.; Desrosiers, M. Topical Probiotics as a Therapeutic Alternative for Chronic Rhinosinusitis: A Preclinical Proof of Concept. Am. J. Rhinol. Allergy 2016, 30, e202–e205. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining The Microbiota for Microbial and Metabolite-Based Immunotherapies. Nat. Rev. Immunol. 2019. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and Molecular Biology of Airway Mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Boucher, R.C. Mucus Clearance as a Primary Innate Defense Mechanism for Mammalian Airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.Q.; Goldstein, N.; Yang, H.; Cowan, A.T.; Chen, B.; Zheng, C.; Palmer, J.N.; Kreindler, J.L.; Cohen, N.A. Inherent Differences in Nasal and Tracheal Ciliary Function in Response to Pseudomonas aeruginosa Challenge. Am. J. Rhinol. Allergy 2011, 25, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.C.; Cope, E.; Chen, B.; Leid, J.G.; Cohen, N.A. Regulation of Murine Sinonasal Cilia Function by Microbial Secreted Factors. Int. Forum Allergy Rhinol. 2012, 2, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cervin, A.; Magnusdottir, A.B.; Runer, T.; Lindberg, S.; Carlén, B.; Forsgren, A.; Magnusdottir, A.B.; Lindberg, S.; Runer, T. Effects on the Ciliated Epithelium of Protein D—Producing and —Nonproducing Nontypeable Haemophilus influenzae in Nasopharyngeal Tissue Cultures. J. Infect. Dis. 1999, 180, 737–746. [Google Scholar]
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic Rhinosinusitis Pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The Tight Junction: A Multifunctional Complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Roscioli, E.; Murphy, J.; Ou, J.; Bassiouni, A.; Wormald, P.J.; Vreugde, S. Staphylococcus Aureus Impairs the Airway Epithelial Barrier In Vitro. Int. Forum Allergy Rhinol. 2015, 5, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Obata, K.; Keira, T.; Miyata, R.; Hirakawa, S.; Takano, K.; Kohno, T.; Sawada, N.; Himi, T.; Kojima, T. Pseudomonas Aeruginosa Elastase Causes Transient Disruption of Tight Junctions and Downregulation of PAR-2 in Human Nasal Epithelial Cells. Respir. Res. 2014, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective Epithelial Barrier in Chronic Rhinosinusitis: The Regulation of Tight Junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, A.; Kiss, M.; Kadocsa, E.; Polyanka, H.; Szabo, K.; Razga, Z.; Bella, Z.; Tiszlavicz, L.; Kemeny, L. Different Activations of Toll-Like Receptors and Antimicrobial Peptides in Chronic Rhinosinusitis with or without Nasal Polyposis. Eur. Arch. Otorhinolaryngol. 2016, 273, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of TH Cytokine Profiles in Patients with Chronic Rhinosinusitis: A Multicenter Study in Europe, Asia, And Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef]
- Chen, M.; Guo, Z.; Ju, W.; Ryffel, B.; He, X.; Zheng, S.G. The Development and Function of Follicular Helper T Cells in Immune Responses. Cell. Mol. Immunol. 2012, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, C. The Biology and Functions of Th22 Cells. In T Helper Cell Differentiation and Their Function; Sun, B., Ed.; Springer: Dodrecht, the Netherlands, 2014; pp. 209–230. [Google Scholar]
- Murphy, K. Janeway’s Immunobiology, 8th ed.; Garland Science, Taylor & Francis Group: New York, NY, USA, 2012; pp. 360–366. [Google Scholar]
- Van Zele, T.; Claeys, S.; Gevaert, P.; van Maele, G.; Holtappels, G.; van Cauwenberge, P.; Bachert, C. Differentiation of Chronic Sinus Diseases by Measurement of Inflammatory Mediators. Allergy 2006, 61, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Van Bruaene, N.; Pérez-Novo, C.A.; Basinski, T.M.; van Zele, T.; Holtappels, G.; de Ruyck, N.; Schmidt-Weber, C.; Akdis, C.; van Cauwenberge, P.; Bachert, C.; et al. T-Cell Regulation in Chronic Paranasal Sinus Disease. J. Allergy Clin. Immunol. 2008, 121, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.P.; Li, H.B.; Wang, B.F.; Wang, S.B.; You, X.J.; Cui, Y.H.; Wang, D.Y.; Desrosiers, M.; Liu, Z. Distinct Immunopathologic Characteristics of Various Types of Chronic Rhinosinusitis in Adult Chinese. J. Allergy Clin. Immunol. 2009, 124, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.S.; Han, D.M.; Sy, C.; Huang, Q.; Sun, Y.; Fan, E.Z.; Li, Y.; Zhou, B. Differential Expression of Toll-Like Receptor Pathway Genes in Chronic Rhinosinusitis with or without Nasal Polyps. Acta Otolaryngol. 2013, 133, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Stevens, W.W.; Peters, A.T.; Suh, L.A.; Norton, J.; Carter, R.G.; Hulse, K.E.; Harris, K.E.; et al. Heterogeneous Inflammatory Patterns in Chronic Rhinosinusitis without Nasal Polyps in Chicago, Illinois. J. Allergy Clin. Immunol. 2017, 139, 699–703. [Google Scholar] [CrossRef]
- Stevens, W.W.; Ocampo, C.J.; Berdnikovs, S.; Sakashita, M.; Mahdavinia, M.; Suh, L.; Takabayashi, T.; Norton, J.E.; Hulse, K.E.; Conley, D.B.; et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Van Zele, T.; Perez-Novo, C.; van Bruaene, N.; Holtappels, G.; DeRuyck, N.; van Cauwenberge, P.; Bachert, C. Different Types of T-Effector Cells Orchestrate Mucosal Inflammation in Chronic Sinus Disease. J. Allergy Clin. Immunol. 2008, 122, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Licona-Limón, P.; Kim, L.K.; Palm, N.W.; Flavell, R.A. TH2, Allergy and Group 2 Innate Lymphoid Cells. Nat. Immunol. 2013, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 Induces IL-4, IL-5, and IL-13 and Th2-Associated Pathologies In Vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Omori, M.; Ziegler, S. Induction of IL-4 Expression in CD4(+) T Cells by Thymic Stromal Lymphopoietin. J. Immunol. 2007, 178, 1396–1404. [Google Scholar] [CrossRef]
- Shaw, J.L.; Fakhri, S.; Citardi, M.J.; Porter, P.C.; Corry, D.B.; Kheradmand, F.; Liu, Y.J.; Luong, A. IL-33–Responsive Innate Lymphoid Cells Are an Important Source of IL-13 in Chronic Rhinosinusitis with Nasal Polyps. Am. J. Respir. Crit. Care Med. 2013, 188, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Favoreto, S.; Avila, P.C.; Schleimer, R.P. TLR3- and Th2 Cytokine-Dependent Production of Thymic Stromal Lymphopoietin in Human Airway Epithelial Cells. J. Immunol. 2007, 179, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, D.L.; Leung, D.Y.M.; Wood, R.; Cunningham, L.; Bean, D.K.; Yasruel, Z.; Schotman, E.; Hamid, Q. Evidence for Distinct Cytokine Expression in Allergic Versus Nonallergic Chronic Sinusitis. J. Allergy Clin. Immunol. 1995, 96, 537–544. [Google Scholar] [CrossRef]
- Zhang, Y.; Derycke, L.; Holtappels, G.; Wang, X.D.; Zhang, L.; Bachert, C.; Zhang, N. Th2 Cytokines Orchestrate the Secretion Of MUC5AC and MUC5B in IL-5-Positive Chronic Rhinosinusitis with Nasal Polyps. Allergy 2018, 74, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Lu, X.; Purkey, M.R.; Homma, T.; Choi, A.W.; Carter, R.; Suh, L.; Norton, J.; Harris, K.E.; Conley, D.B.; et al. Increased Expression of the Epithelial Anion Transporter Pendrin/SLC26A4 in Nasal Polyps of Patients with Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2015, 136, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, C. Oxygen Matters: Hypoxia as a Pathogenic Mechanism in Rhinosinusitis. BMB Rep. 2018, 51, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Zhang, N.; Holtappels, G.; Ruyck, N.D.; Krysko, O.; Crombruggen, K.V.; Braun, H.; Johnston, S.L.; Papadopoulos, N.G.; Zhang, L.; et al. Staphylococcus aureus Induces a Mucosal Type 2 Immune Response via Epithelial Cell–derived Cytokines. Am. J. Respir. Crit. Care Med. 2018, 198, 452–463. [Google Scholar] [PubMed]
- Bachert, C.; van Kempen, M.J.P.K.; Höpken, K.; Holtappels, G.; Wagenmann, M. Elevated Levels of Myeloperoxidase, Pro-Inflammatory Cytokines and Chemokines in Naturally Acquired Upper Respiratory Tract Infections. Eur. Arch. Otorhinolaryngol. 2001, 258, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, D.L. Drivers of Chronic Rhinosinusitis: Inflammation Versus Infection. J. Allergy Clin. Immunol. 2015, 136, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Gratz, I.K.; Rosenblum, M.D.; Abbas, A.K. The Life of Regulatory T Cells. Ann. N. Y. Acad. Sci. 2013, 1283, 8–12. [Google Scholar] [CrossRef]
- Van Bruaene, N.; Derycke, L.; Perez-Novo, C.A.; Gevaert, P.; Holtappels, G.; De Ruyck, N.; Cuvelier, C.; Van Cauwenberge, P.; Bachert, C. TGF-Beta Signaling and Collagen Deposition in Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2009, 124, 253. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, M.; Hassan, T.; Chotirmall, S.H. Transforming Growth Factor B and Severe Asthma: A Perfect Storm. Respir. Med. 2014, 108, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. Tgfbeta in The Context of an Inflammatory Cytokine Milieu Supports De Novo Differentiation of IL-17-Producing T Cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory Endotypes of Chronic Rhinosinusitis Based on Cluster Analysis of Biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Akdis, C.A. Phenotypes and Emerging Endotypes of Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2016, 4, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Zhang, N.; Hellings, P.W.; Bousquet, J. Endotype-Driven Care Pathways in Patients with Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2018, 141, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Koennecke, M.; Klimek, L.; Mullol, J.; Gevaert, P.; Wollenberg, B. Subtyping of Polyposis Nasi: Phenotypes, Endotypes and Comorbidities. Allergo J. Int. 2018, 27, 56–65. [Google Scholar] [CrossRef]
- Liao, B.; Liu, J.X.; Li, Z.Y.; Zhen, Z.; Cao, P.P.; Yao, Y.; Long, X.B.; Wang, H.; Wang, Y.; Schleimer, R.; et al. Multidimensional Endotypes of Chronic Rhinosinusitis and their Association with Treatment Outcomes. Allergy 2018, 73, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Klingler, A.I.; Stevens, W.W.; Peters, A.T.; Poposki, J.A.; Suh, L.; Norton, J.; Carter, R.G.; Hulse, K.E.; Harris, K.E.; et al. Heterogenous Inflammation in Chronic Rhinosinusitis without Nasal Polyps. J. Allergy Clin. Immunol. 2016, 137, AB285. [Google Scholar] [CrossRef]
- Kato, A. Immunopathology of Chronic Rhinosinusitis. Allergol. Int. 2015, 64, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Soler, Z.M.; Hyer, J.M.; Ramakrishnan, V.; Smith, T.L.; Mace, J.; Rudmik, L.; Schlosser, R.J. Identification of Chronic Rhinosinusitis Phenotypes Using Cluster Analysis. Int. Forum Allergy Rhinol. 2015, 5, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Soler, Z.M.; Hyer, J.M.; Rudmik, L.; Ramakrishnan, V.; Smith, T.L.; Schlosser, R.J. Cluster Analysis and Prediction of Treatment Outcomes for Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2016, 137, 1054–1062. [Google Scholar] [CrossRef]
- Cope, E.K.; Goldberg, A.N.; Pletcher, S.D.; Lynch, S.V. Compositionally and Functionally Distinct Sinus Microbiota in Chronic Rhinosinusitis Patients Have Immunological and Clinically Divergent Consequences. Microbiome 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, M.; Waldvogel-Thurlow, S.; Zoing, M.; Chang, K.; Radcliff, F.J.; Wagner Mackenzie, B.; Biswas, K.; Douglas, R.G.; Taylor, M.W. Inflammatory Endotypes and Microbial Associations in Chronic Rhinosinusitis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Turner, J.H.; Chandra, R.K.; Li, P.; Bonnet, K.; Schlundt, D.G. Identification of Clinically Relevant Chronic Rhinosinusitis Endotypes Using Cluster Analysis of Mucus Cytokines. J. Allergy Clin. Immunol. 2018, 141, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; van Cauwenberge, P.; et al. Omalizumab is Effective in Allergic and Non-allergic Patients with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. 2012, 129. [Google Scholar] [CrossRef]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced Need for Surgery in Severe Nasal Polyposis with Mepolizumab: Randomized Trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031. [Google Scholar] [CrossRef]
- Gevaert, P.; Van Bruaene, N.; Cattaert, T.; Van Steen, K.; Van Zele, T.; Acke, F.; De Ruyck, N.; Blomme, K.; Sousa, A.R.; Marshall, R.P.; et al. Mepolizumab, a Humanized Anti-IL-5 Mab, as a Treatment Option for Severe Nasal Polyposis. J. Allergy Clin. Immunol. 2011, 128, 989–995. [Google Scholar] [CrossRef]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect Of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients with Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Silfvast-Kaiser, A.; Paek, S.Y.; Menter, A. Anti-IL17 Therapies for Psoriasis. Expert. Opin. Biol. Ther. 2019, 19, 45–54. [Google Scholar] [CrossRef]
- Fleischmann, R. Anakinra in the Treatment of Rheumatic Disease. Expert. Opin. Biol. Ther. 2006, 2, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lacy, S.E.; Wu, C.; Ambrosi, D.J.; Hsieh, C.M.; Bose, S.; Miller, R.; Conlon, D.M.; Tarcsa, E.; Chari, R.; Ghayur, T.; et al. Generation and Characterization of ABT-981, a Dual Variable Domain Immunoglobulin (DVD-Ig(TM)) Molecule That Specifically and Potently Neutralizes Both IL-1alpha And IL-1beta. mAbs 2015, 7, 605–619. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahern, S.; Cervin, A. Inflammation and Endotyping in Chronic Rhinosinusitis—A Paradigm Shift. Medicina 2019, 55, 95. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55040095
Ahern S, Cervin A. Inflammation and Endotyping in Chronic Rhinosinusitis—A Paradigm Shift. Medicina. 2019; 55(4):95. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55040095
Chicago/Turabian StyleAhern, Sinead, and Anders Cervin. 2019. "Inflammation and Endotyping in Chronic Rhinosinusitis—A Paradigm Shift" Medicina 55, no. 4: 95. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55040095




