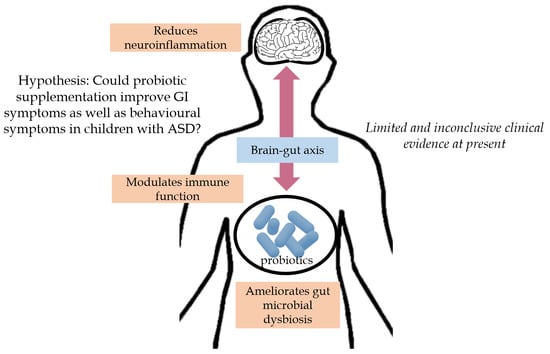
A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Packer, A. Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci. Biobehav. Rev. 2016, 64, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; D’Mello, S.R.; Narayanan, V. Epigenetics, autism spectrum, and neurodevelopmental disorders. Neurotherapeutics 2013, 10, 742–756. [Google Scholar] [CrossRef]
- Ollendick, T.H.; White, S.W. The presentation and classification of anxiety in autism spectrum disorder: Where to from here? Clin. Psychol. Sci. Pract. 2012, 19, 352–355. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J.; Furuta, G.T.; Levy, J.; VandeWater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 2010, 125 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rossignol, D.A. Identification and treatment of pathophysiological comorbidities of autism spectrum disorder to achieve optimal outcomes. Clin. Med. Insights Pediatr. 2016, 10, 43–56. [Google Scholar] [CrossRef]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [Green Version]
- Finegold, S.M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; Van Der Sluis, M.; Bouma, J.; Boehm, G.; Van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [PubMed]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, A. Probiotics in the treatment of irritable bowel syndrome. J. Clin. Gastroenterol. 2004, 38, S104–S106. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Peters, C.; Ho, C.Y.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Neufeld, K.A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.; Venkatanarayanan, N.; Ho, C.Y.; Lim, D.Y.; Yeo, W.S. A systematic review of the effects of probiotics on schizophrenia symptoms. Neuropsychobiology. 2019, 78, 1–6. [Google Scholar] [CrossRef]
- Li, Y.J.; Ou, J.J.; Li, Y.M.; Xiang, D.X. Dietary supplement for core symptoms of autism spectrum disorder: Where are we now and where should we go? Front. Psychiatry 2017, 8, 155. [Google Scholar] [CrossRef]
- Grossi, E.; Melli, S.; Dunca, D.; Terruzzi, V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med. Case Rep. 2016, 4, 2050313X16666231. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Gibson, E.L.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Błaszczyk, S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012, 28, 124–126. [Google Scholar] [CrossRef]
- Parracho, H.M.; Gibson, G.R.; Knott, F.; Bosscher, D.; Kleerebezem, M.; McCartney, A.L. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiotics Prebiotics 2010, 5, 69–74. [Google Scholar]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Kang, J.; Van Zyl, N.; Barthow, C.; Wickens, K.; Stanley, T.; Coomarasamy, C.; Purdie, G.; Murphy, R.; Crane, J.; et al. Effect of early probiotic supplementation on childhood cognition, behaviour and mood a randomised, placebo-controlled trial. Acta Paediatr. 2018, 107, 2173–2178. [Google Scholar] [CrossRef]
- West, R.; Roberts, E.; Sichel, L.S.; Sichel, J. Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the Delpro® Probiotic and immunomodulator formulation. J. Prob. Health 2013, 1, 102. [Google Scholar]
- Romeo, M.G.; Romeo, D.M.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011, 31, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.; Loke, W.; Lim, D.Y.; Yeo, W.S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.; Hagerman, R.J. The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Hughes, H.K.; Rose, D.; Ashwood, P. The gut microbiota and dysbiosis in Autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Shahrestani, S.; Kemp, A.H.; Guastella, A.J. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: A meta-analysis. Neuropsychopharmacology 2013, 38, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Pelto, L.; Isolauri, E.; Lilius, E.M.; Nuutila, J.; Salminen, S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1998, 28, 1474–1479. [Google Scholar] [CrossRef]
- Williams, K.A.; Swedo, S.E. Post-infectious autoimmune disorders: Sydenham’s chorea, PANDAS and beyond. Brain Res. 2015, 1617, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ng, Q.X.; Zhang, B.; Wei, Z.K.; Hassan, M.; He, Y.; Ong, C.N. Employing multi-omics to elucidate the hormetic response against oxidative stress exerted by nC60 on Daphnia pulex. Environ. Pollut. 2019, 251, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, B.; He, Y.; Hu, C.; Ng, Q.X.; Zhang, H.; Ong, C.N. Biological effect of aqueous C60 aggregates on Scenedesmus obliquus revealed by transcriptomics and non-targeted metabolomics. J. Hazard. Mater. 2017, 324, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Persico, A.M.; Napolioni, V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol. Teratol. 2013, 36, 82–90. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.P.; Cain, D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef]
- Vamanu, E. Complementary Functional Strategy for Modulation of Human Gut Microbiota. Curr. Pharm. Des. 2018, 24, 4144–4149. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J. Strain-Specificity and Disease-Specificity of Probiotic efficacy: A Systematic Review and meta-analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.; Ruelaz, A.; Wang, Z.; Miles, J.N.; Suttorp, M.J.; Johnsen, B.; Shanman, R.; Slusser, W.; Fu, N.; et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid. Rep. Technol. Assess. 2011, 200, 1–645. [Google Scholar]
- Gwee, K.A.; Lee, W.W.; Ling, K.L.; Ooi, C.J.; Quak, S.H.; Dan, Y.Y.; Siah, K.T.; Huang, J.G.; Chua, A.S.; Hilmi, I.N.; et al. Consensus and contentious statements on the use of probiotics in clinical practice: A south east Asian gastro-neuro motility association working team report. J. Gastroenterol. Hepatol. 2018, 33, 1707–1716. [Google Scholar] [CrossRef]
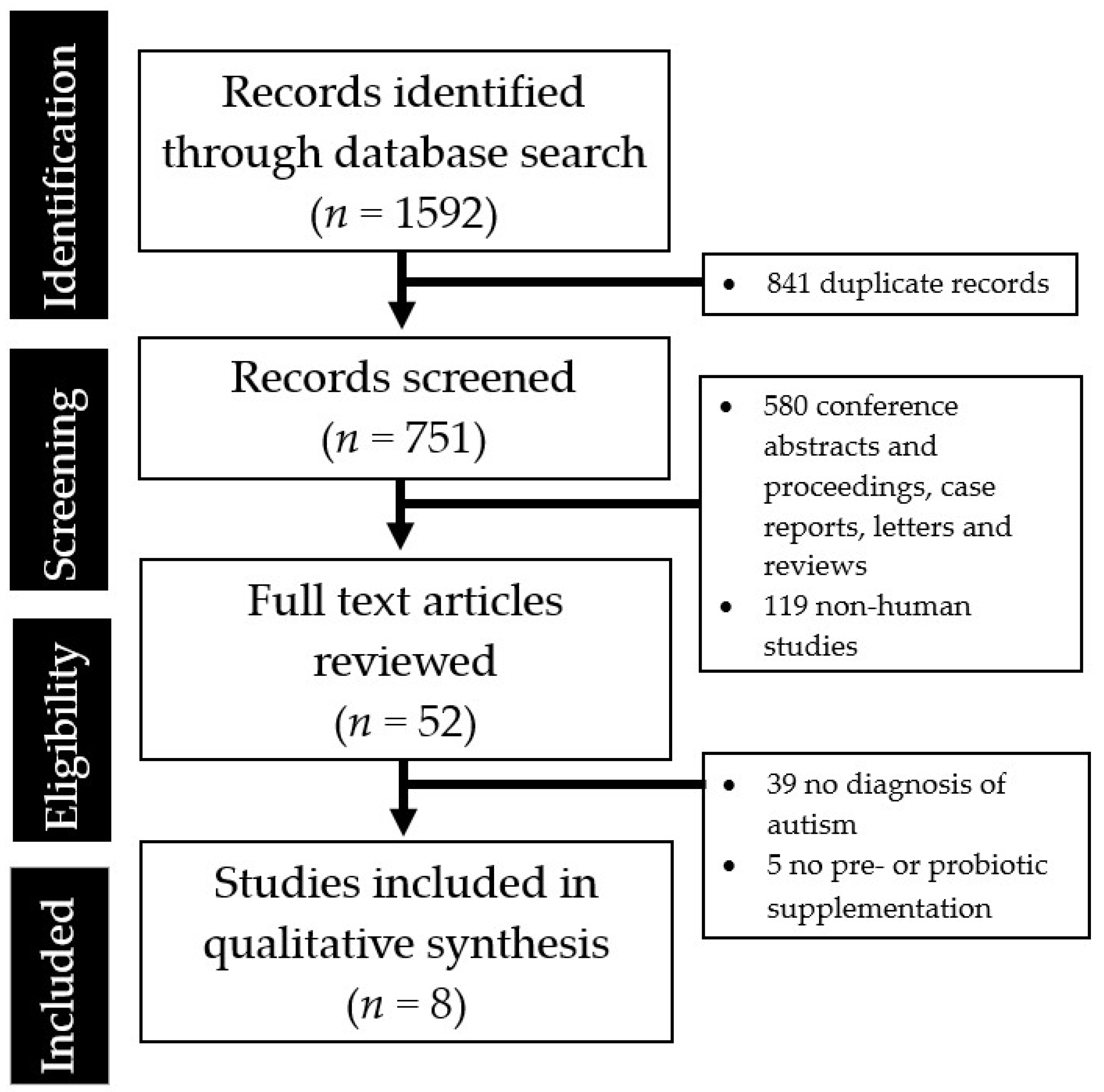
| Author, Year | Study Design | Sample Size (N) | Study Population | Prebiotic or Probiotics Strains | Study Duration | Key Findings |
|---|---|---|---|---|---|---|
| Prebiotics | ||||||
| Grimaldi 2018 [23] | Randomized, double-blind, placebo-controlled | 41 | 4–11 year old children with ASD, 75% male, UK | Maltodextrin— GLUCIDEX®; 1.8 g | 6 weeks |
|
| Sanctuary 2019 [24] | Randomized, double-blind, cross over study | 8 | 2–11 year old children with ASD, 87.5% male, US | Bovine colostrum product (BCP) Bifidobacterium longum supbsp. infantis (UCD272) | Once daily for 12 weeks |
|
| Probiotics | ||||||
| Kaluzna-Czaplinska 2012 [25] | Prospective, open-label | 22 | 4–10 year old children with ASD, 90% male, Poland | Lactobacillus acidophilus | Twice daily for 1 month |
|
| Parracho 2010 [26] | Randomized, double-blind, placebo-controlled | 39 | 4–16 year old children with ASD, UK | Lactobacillus plantarum | 3 weeks |
|
| Shaaban 2018 [27] | Prospective, open-label | 30 | 5–9 year old children with ASD, 63% male, Egypt | Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacteria longum | Once daily for 3 months |
|
| Slykerman 2018 [28] | Two-center, randomized, double-blind, placebo-controlled | 342 | Children followed from birth to 11 years, New Zealand | Lactobacillus rhamnosus, Bifidobacteria animalis, Bifidobacterium lactis HN019 | Mothers given probiotics from 35 weeks pregnant until 6 months. Children receive treatment from birth to 2 years |
|
| Tomova 2015 [12] | Prospective, open-label, controlled | 29 | Children with ASD from 2–9 years old, siblings of ASD children 5–17 years old, control children 2–11 years old, Slovakia | 3 strains of Lactobacillus, 2 strains of Bifidobacteria, 1 strain of Streptococcus | 3 times daily for 4 months |
|
| West 2013 [29] | Prospective, open-label | 33 | 3–16 year old children with ASD, USA | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii, Bifidobacteria longum, Bifidobacteria bifidum | 3 times daily for 21 days |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55050129
Ng QX, Loke W, Venkatanarayanan N, Lim DY, Soh AYS, Yeo WS. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina. 2019; 55(5):129. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55050129
Chicago/Turabian StyleNg, Qin Xiang, Wayren Loke, Nandini Venkatanarayanan, Donovan Yutong Lim, Alex Yu Sen Soh, and Wee Song Yeo. 2019. "A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders" Medicina 55, no. 5: 129. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55050129