Advanced Drug-Eluting Poly (Vinyl Chloride) Surfaces Deposited by Spin Coating
Abstract
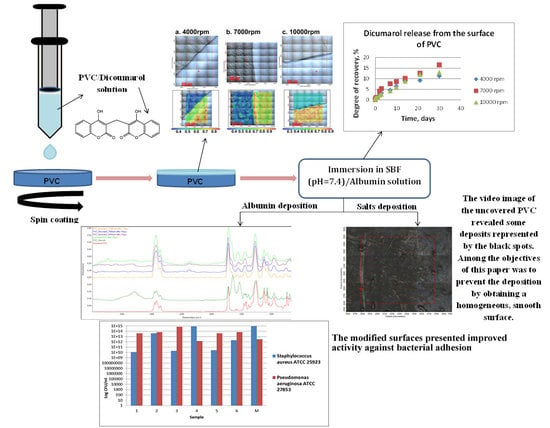
:1. Introduction
2. Materials and Methods
2.1. Dicoumarol Synthesis
2.2. PVC/Dicoumarol Film Deposition
2.2.1. Experimental Procedure
2.2.2. Quantitative Assessment of the Selected Strains Capacity to Adhere to the PVC Surfaces
3. Results
3.1. Release of Dicoumarol from the Thin Film Deposited on the Surface of PVC
3.2. Protein Adsorption on the Surface of PVC
3.3. Bacterial Adhesion
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gehrke, S.H.; Fisher, J.P.; McBride, J.F.; O’Connor, S.M.; Zhu, H. Gel-coated catheters as drug delivery systems. J. Am. Chem. Soc. 1999, 43–53. [Google Scholar] [CrossRef]
- Randolph, A.G.; Cook, D.J.; Gonzales, C.A.; Andrew, M. Benefit of Heparin in Central Venous and Pulmonary Artery Catheters. Chest 1998, 113, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.Y.; Ivany, J.N.; Perkovic, V.; Gallagher, M.P.; Jardine, M.J. Anticoagulant therapies for the prevention of intravascular catheters malfunction in patients undergoinghaemodialysis: Systematic review and meta-analysis of randomized, controlled trials. Nephrol. Dial. Transplant. 2013, 28, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.O.; Hinchliffe, R.; Loftus, I.M.; Thompson, M.M.; Holt, P.J. Indications for Catheter-Directed Thrombolysis in the Management of Acute Proximal Deep Venous Thrombosis. Arter. Thromb. Vasc. Boil. 2010, 30, 669–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, M.; Navarro, R.; Grohens, Y.; Reinecke, H.; Mijangos, C. Controlled wet-chemical modification and bacterial adhesion on PVC-surfaces. Polym. Degrad. Stab. 2006, 91, 1915–1918. [Google Scholar] [CrossRef]
- Merchan, M.; Sedlarikova, J.; Vesel, A.; Machovsky, M.; Sedlarik, V.; Saha, P. Antimicrobial silver nitrate-doped polyvinyl chloride cast films: influence of solvent on morphology and mechanical properties. Int. J. Polym. Mater. 2012, 62, 101–108. [Google Scholar] [CrossRef]
- Sowe, M.; Polaskova, M.; Kuritka, I.; Sedlacek, T.; Merchan, M. Analysis of Antibacterial Action of Polyvinyl Chloride Surface Modified with Gentian Violet. Int. J. Polym. Anal. Charact. 2009, 14, 678–685. [Google Scholar] [CrossRef]
- Sheth, N.K.; Franson, T.R.; Rose, H.D.; Buckmire, F.L.; Cooper, J.A.; Sohnle, P.G. Colonization of bacteria on polyvinyl chloride and teflon intravascular catheters in hospital patients. J. Clin. Microbiol. 1983, 18, 1061–1063. [Google Scholar]
- Pai, M.; Crowther, M.A. Anticoagulation for the prevention of central venous catheter associated-thrombosis: an evidence based commentary. Pol. Arch. Med. Wewn. 2007, 117, 494. [Google Scholar] [CrossRef]
- Mikušová, N.; Nechvilova, K.; Kalendova, A.; Hájková, T.; Capakova, Z.; Junkar, I.; Lehocký, M.; Mozetič, M.; Humpolíček, P. The effect of composition of a polymeric coating on the biofilm formation of bacteria and filamentous fungi. Int. J. Polym. Mater. 2018, 68, 152–159. [Google Scholar] [CrossRef]
- De Mel, A.; Cousins, B.G.; Seifalian, A.M. Surface Modification of Biomaterials: A Quest for Blood Compatibility. Int. J. Biomater. 2012, 2012, 707863. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Lăcătuș, R.; Papuc, I.; Purdoiu, R.C.; Păvăloiu, A.; Gal, A.; Antal, I.Z. Radiological diagnosis in experimental rabbit endocarditis. Sci. Work. Univ. Agron. Sci. Vet. Med. Buchar. C Vet. Med. 2010, 56, 186–195. [Google Scholar]
- Purdoiu, R.C.; Papuc, I.; Lăcătuş, R.; Păvăloiu, A.N. Radiological Diagnosis in Heart Conditions in Dogs. Cluj Vet. J. 2011, 1, 19. [Google Scholar]
- D’Agostino, R.; Favia, P.; Fracassi, F. Plasma Processing of Polymers; Springer Science & Business Media: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Xie, Y.; Yang, Q. Surface modification of poly (vinyl chloride) for antithrombogenicity study. J. Appl. Polym. Sci. 2002, 85, 1013–1018. [Google Scholar] [CrossRef]
- Kizhakkedathu, J.N.; Zou, Y.; Phani, A.S.; Brooks, D.E. Shape Memory Materials by Surface Modification. US Patent 9962521, 8 May 2018. [Google Scholar]
- Singh, J.; Agrawal, K.K. Modification of poly (vinyl chloride) for biocompatibility improvement and biomedical application-review. Polym Plast. Technol. Eng. 1992, 31, 203–212. [Google Scholar] [CrossRef]
- Niemczyk, A.; Kaczorowski, P.; El Fray, M. Spin-coated chitosan on copolyester substrates. Prog. Chem. Appl. Chitin Deriv. 2015, 20, 236–245. [Google Scholar] [CrossRef]
- Birnie, D.P. Spin Coating Technique. In Sol-Gel Technologies for Glass Producers and Users; Michel, A., Aegerter, M.M., Eds.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Hall, D.B.; Underhill, P.; Torkelson, J.M. Spin Coating of Thin and Ultrathin Polymer Films. Polym. Eng. Sci. 1998, 38, 2039–2045. [Google Scholar] [CrossRef]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental understanding and modeling of spin coating process: A review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Fereidoon, A.; Katouzian, S.; Taraghi, I.; Paszkiewicz, S. Nanomechanical and nanoscratch performance of polystyrene/poly (methyl methacrylate)/multi-walled carbon nanotubes nanocomposite coating. Polym. Compos. 2017, 39, E962–E968. [Google Scholar] [CrossRef]
- Zodrow, K.R.; Schiffman, J.D.; Elimelech, M. Biodegradable Polymer (PLGA) Coatings Featuring Cinnamaldehyde and Carvacrol Mitigate Biofilm Formation. Langmuir 2012, 28, 13993–13999. [Google Scholar] [CrossRef] [PubMed]
- Chamsaz, E.A.; Mankoci, S.; Barton, H.A.; Joy, A. Non-toxic Cationic Coumarin Polyester Coatings Prevent Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2017, 9, 6704–6711. [Google Scholar] [CrossRef] [PubMed]
- Gunell, M.; Haapanen, J.; Brobbey, K.J.; Saarinen, J.J.; Toivakka, M.; Mäkelä, J.M.; Huovinen, P.; Eerola, E. Antimicrobial characterization of silver nanoparticle-coated surfaces by “touch test” method. Nanotechnol. Sci. Appl. 2017, 10, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Barbalinardo, M.; Caicci, F.; Cavallini, M.; Gentili, D. Protein Corona Mediated Uptake and Cytotoxicity of Silver Nanoparticles in Mouse Embryonic Fibroblast. Small 2018, 14, 1801219. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Chung, R.J.; Hsieh, M.F.; Huang, C.W.; Perng, L.H.; Wen, H.W.; Chin, T.S. Antimicrobial Effects and Human Gingival Biocompatibility of Hydroxyapatite Sol–Gel Coatings. J. Biomed. Mater. Res. B 2006, 76, 169–178. [Google Scholar] [CrossRef]
- Stobie, N.; Duffy, B.; McCormack, D.E.; Colreavy, J.; Hidalgo, M.; McHale, P.; Hinder, S.J. Hinder Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol–gel coating. Biomaterials 2008, 29, 963–969. [Google Scholar] [CrossRef]
- Sutha, S.; Karunakaran, G.; Rajendran, V. Enhancement of antimicrobial and long-term biostability of the zinc-incorporated hydroxyapatite coated 316L stainless steel implant for biomedical application. Ceram. Int. 2013, 39, 5205–5212. [Google Scholar] [CrossRef]
- Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E.; Borges, F. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar]
- Matos, M.J.; Teran, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. Synthesis and Study of a Series of 3-Arylcoumarins as Potent and Selective Monoamine Oxidase B Inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Iqbal, S.; Lodhi, M.A.; Maharvi, G.M.; Choudhary, M.I.; Perveen, S. Biscoumarin: New class of urease inhibitors; economical synthesis and activity. Bioorganic Med. Chem. 2004, 12, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, S.; Karami, B.; Eskandari, K.; Hoseini, S.J.; Nasrabadi, H. Convenient on water synthesis of novel derivatives of dicoumarol as functional vitamin K depleter by Fe3O4 magnetic nanoparticles. Arab. J. Chem. 2017, 10, S3907–S3912. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Karami, B.; Eskandari, K.; Rashidi, A. A facile and practical p-toluenesulfonic acid catalyzed route to dicoumarols containing an aryl group. S. Afr. J. Chem. 2015, 68, 53–56. [Google Scholar] [CrossRef]
- Prabhakar, M. EDTA-catalyzed fast and efficient eco-friendly synthesis of dicoumarol derivatives in water. J. Chem. Pharm. Res. 2013, 5, 89–93. [Google Scholar]
- Burrows, A.; Holman, J.; Parsons, A.; Pilling, G.; Price, G. Chemistry 3: Introducing Inorganic, Organic and Physical Chemistry; Oxford University Press: Oxford, UK, 2017; pp. 319–320. [Google Scholar]
- Thibodeau, G.A.; Patton, K.T. Acid-Base Balance. In Structure & Function of the Body; Elsevier: Amsterdam, The Netherlands, 2016; pp. 427–438. [Google Scholar]
- Tas, A.C. Use of biomineralization media in biomimetic synthesis of hard tissue substitutes. Adv. Bioceram. Biotechnol. II 2014, 247, 91–104. [Google Scholar]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A 2003, 65, 188–195. [Google Scholar] [CrossRef]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazǎr, V.; Chifiriuc, M.C.; Lazar, V. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690. [Google Scholar] [CrossRef]
- Cotar, A.I.; Grumezescu, A.M.; Andronescu, E.; Voicu, G.; Ficai, A.; Ou, K.L.; Huang, K.S.; Chifiriuc, M.C. Nanotechnological solution for improving the antibiotic efficiency against biofilms developed by gram-negative bacterial strains. Lett. Appl. NanoBioSci. 2013, 2, 97–104. [Google Scholar]
- Garten, S.; Wosilait, W.D. Comparative study of the binding of coumarin anticoagulants and serum albumins. Biochem. Pharmacol. 1971, 20, 1661–1668. [Google Scholar] [CrossRef]
- Karakeçılı, A.G.; Gümüşderelıoğlu, M. Comparison of bacterial and tissue cell initial adhesion on hyzdrophilic/hydrophobic biomaterials. J. Biomater. Sci. 2002, 13, 185–196. [Google Scholar] [CrossRef]
- Bulard, E.; Fontaine-Aupart, M.P.; Dubost, H.; Zheng, W.; Bellon-Fontaine, M.N.; Herry, J.M.; Bourguignon, B. Competition of Bovine Serum Albumin Adsorbtion and Bacterial Adhesion onto Surface-Grafted ODT: In situ study by Vibrational SFG and Fluorescence Confocal Microscopy. Langmuir 2012, 28, 17001–17010. [Google Scholar] [CrossRef] [PubMed]
- Khelissa, S.O.; Abdallah, M.; Jama, C.; Faille, C.; Chihib, N.E. Bacterial contamination and biofilm formation on abiotic surfaces and strategies to overcome their persistance. J. Mater. Environ. Sci. 2017, 8, 3326–3346. [Google Scholar]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Fabre, H.; Mercier, D.; Galtayries, A.; Portet, D.; Delorme, N.; Bardeau, J.-F. Impact of hydrophilic and hydrophobic functionalization of flat TiO2/Ti surfaces on proteins adsorption. Appl. Surf. Sci. 2018, 432, 15–21. [Google Scholar] [CrossRef]
- Nagaoka, S.; Kawakami, H. Inhibition of Bacterial Adhesion and Biofilm Formation by a Heparinized Hydrophilic Polymer. ASAIO J. 1995, 41, M365–M368. [Google Scholar] [CrossRef]
- Bürgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef]
- Herrero, M.; Quéméner, E.; Ulve, S.; Reinecke, H.; Mijangos, C.; Grohens, Y. Bacterial adhesion to poly (vinyl chloride) films: Effect of chemical modification and water induced surface recontruction. J. Adhes. Sci. Technol. 2006, 20, 183–195. [Google Scholar] [CrossRef]
- Lutz, B.R.; Fulton, G.P.; Akers, R.P. White Thromboembolism in the Hamster Chelk Pouch after Trauma, Infection and Neoplasia. Circulation 1951, 3, 339–351. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novák, I.; Lehocký, M.; Sedlarik, V.; Vesel, A.; Junkar, I.; Sáha, P.; Chodák, I. An in vitro bacterial adhesion assessment of surface-modified medical-grade PVC. Colloids Surf. B Biointerfaces 2010, 77, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, T.; Das, M.C.; Das, A.; Bhowmik, S.; Sandhu, P.; Akhter, Y.; Bhattacharjee, S.; De, U.C. Modulation of S. aureus and P. aeruginosa biofilm: An in vitro study with new coumarin derivatives. World J. Microbiol. Biotechnol. 2018, 34, 170. [Google Scholar] [CrossRef] [PubMed]
- Petnapapun, K.; Chavasiri, W.; Sompornpisut, P. Structure-Activity Relationships of 3,3′-Phenylmethylene-bis-4-hydroxycoumarins: Selective and Potent Inhibitors of Gram-Positive Bacteria. Sci. World J. 2013, 2013, 178649. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ikram, M.; Baker, R.J.; Zubair, M.; Azad, E.; Min, S.; Riaz, K.; Mok, K.H.; Rehman, S.-U. Synthesis, characterization, in vitro antimicrobial, and U2OS tumoricidal activities of different coumarin derivatives. Chem. Cent. J. 2013, 7, 68. [Google Scholar] [CrossRef] [PubMed]
















| Nr. Crt | Dispencer | Spread | EBR | Dry |
|---|---|---|---|---|
| 11 | Spin acceleration: 1000 rpm | Spin acceleration: 100 rpm | Spin acceleration: 1000 rpm | Spin acceleration: 1000 rpm |
| Spin speed: 100 rpm | Spin speed: 2000 rpm | Spin speed: 500 rpm | Spin speed: 4000 rpm | |
| Spin time: 5 ss, s | Spin time: 20 ss, s | Spin time: 10 ss, s | Spin time: 20 ss, s | |
| 22 | Spin acceleration: 1000 rpm | Spin acceleration: 100 rpm | Spin acceleration: 1000 rpm | Spin acceleration: 1000 rpm |
| Spin speed: 100 rpm | Spin speed: 2000 rpm | Spin speed: 500 rpm | Spin speed: 7000 rpm | |
| Spin time: 5 ss, s | Spin time: 20 ss, s | Spin time: 10 ss, s | Spin time: 20 ss, s | |
| 33 | Spin acceleration: 1000 rpm | Spin acceleration: 100 rpm | Spin acceleration: 1000 rpm | Spin acceleration: 1000 rpm |
| Spin speed: 100 rpm | Spin speed: 2000 rpm | Spin speed: 500 rpm | Spin speed: 10,000 rpm | |
| Spin time: 5 ss, s | Spin time: 20 ss, s | Spin time: 10 ss, s | Spin time: 20 ss, s |
| Sample | Number |
|---|---|
| PVC/dicoumarol 4000 rpm | 1 |
| Albumin adsorbed PVC/dicoumarol 4000 rpm | 2 |
| PVC/dicoumarol 7000 rpm | 3 |
| Albumin adsorbed PVC/dicoumarol 7000 rpm | 4 |
| PVC/dicoumarol 10,000 rpm | 5 |
| Albumin adsorbed PVC/dicoumarol 10,000 rpm | 6 |
| PVC | M |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, O.C.; Maximov, M.; Trusca, R.; Ficai, A.; Ficai, D.; Ilie, C.-I.; Ditu, L.-M.; Andronescu, E. Advanced Drug-Eluting Poly (Vinyl Chloride) Surfaces Deposited by Spin Coating. Medicina 2019, 55, 421. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55080421
Duta OC, Maximov M, Trusca R, Ficai A, Ficai D, Ilie C-I, Ditu L-M, Andronescu E. Advanced Drug-Eluting Poly (Vinyl Chloride) Surfaces Deposited by Spin Coating. Medicina. 2019; 55(8):421. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55080421
Chicago/Turabian StyleDuta, Oana Cristina, Maxim Maximov, Roxana Trusca, Anton Ficai, Denisa Ficai, Cornelia-Ioana Ilie, Lia-Mara Ditu, and Ecaterina Andronescu. 2019. "Advanced Drug-Eluting Poly (Vinyl Chloride) Surfaces Deposited by Spin Coating" Medicina 55, no. 8: 421. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55080421