From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research
Abstract
:1. Introduction
- (1)
- Fasting plasma glucose test (FPG): where fasting refers to the absence of food and drink intake, apart from water, for at least 8 h before the test; or
- (2)
- Oral glucose tolerance test (OGTT): where a patient consumes a glucose syrup solution containing 75 g of glucose before which a blood test is carried out to determine 2-hr plasma glucose (PG); or
- (3)
- A1C (Glycated hemoglobin or hemoglobin bounded to glucose) levels via a laboratory test; or
- (4)
- Random PG of more than or equal to 200 mg/dL or 11.1 mmol/L in patients that displayed symptoms of hyperglycemia or hyperglycemic crisis.
2. Risk Factors of Type II Diabetes
2.1. Evidence for Lifestyle and Environmental Risk Factors
2.1.1. Obesity
2.1.2. Sedentary Lifestyle
2.1.3. Ageing
2.1.4. Sex and Gender
2.1.5. Hypertension
2.1.6. Smoking
2.1.7. Alcohol
2.2. Evidence for Genetic Risk Factors
2.2.1. Family Based Linkage Analysis
2.2.2. The Candidate Gene Approach
2.2.3. Genome Wide Association Studies (GWAS)
3. Novel Methods to Monitor Blood Glucose Level
- Hands should be washed before testing as dirt and residue can lead to inaccuracies.
- Insert a single test strip into the glucose meter.
- A small drop of blood should be obtained by prinking the finger-tip using the lancet (needle on blood sampling device), where the volume of blood should be sufficient in filling the test field.
- Place blood drop onto test strip without smearing it, as smearing can lead to inaccuracies.
- Glucose meter will then display the blood glucose level.
4. Treatment of Pre-Diabetes
4.1. Lifestyle Intervention
4.2. Pharmacological Interventions
4.2.1. Metformin
4.2.2. Thiazolidinediones
4.2.3. α-Glucosidase Inhibitors
4.2.4. Incretins
4.2.5. Sodium-Glucose Cotransporter (SGLT) 2 Inhibitors
4.2.6. Anti-Obesity Drugs
4.3. Bariatric Surgery
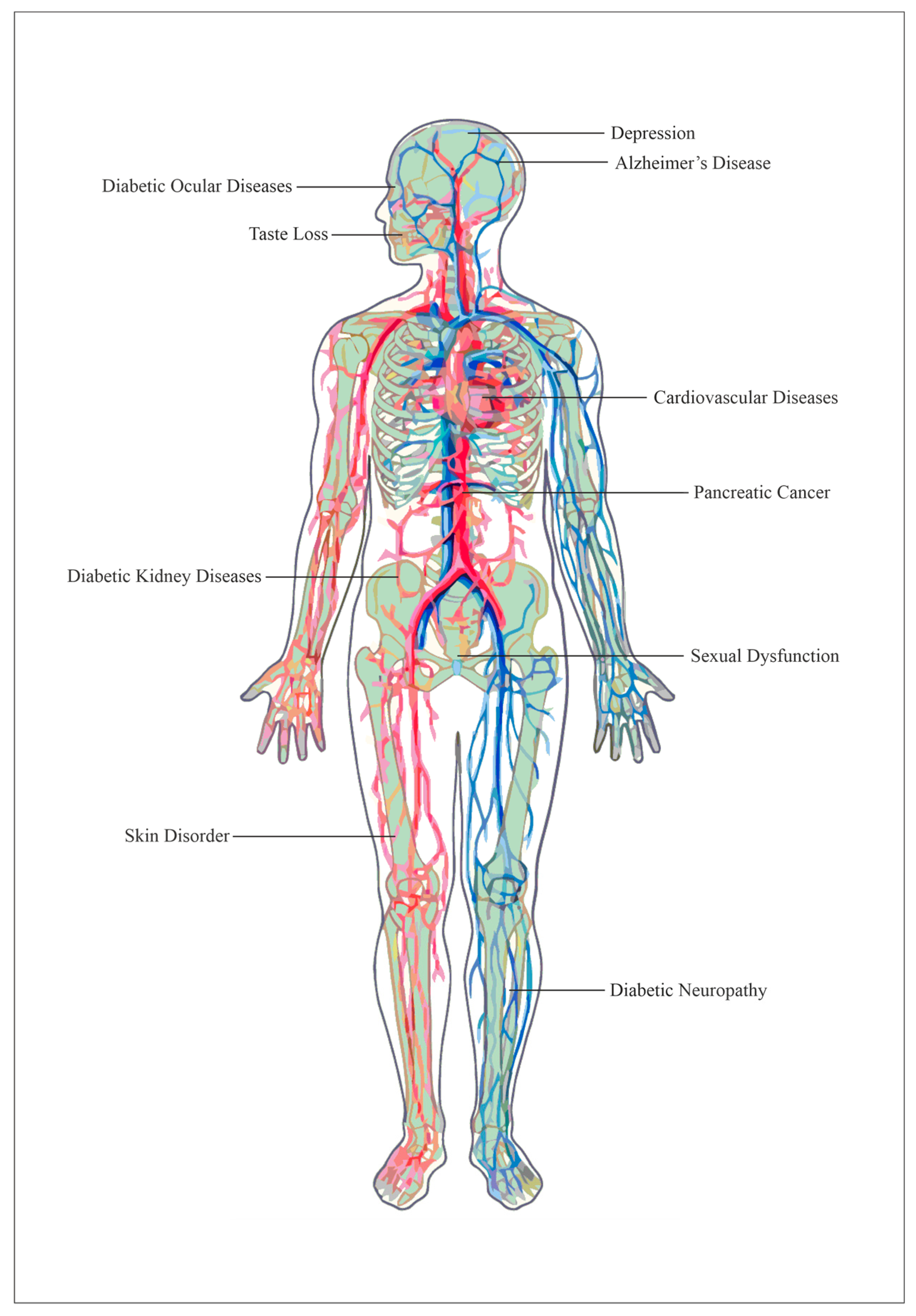
5. Complications
5.1. Cancer
5.2. Depression
5.3. Alzheimer’s Disease
5.4. Diabetic Ocular Diseases
5.5. Taste Loss
5.6. Cardiovascular Diseases
5.7. Diabetic Kidney Diseases
5.8. Sexual Dysfunction
5.9. Skin Disorder
5.10. Diabetic Neuropathy
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Edgerton, D.S.; Kraft, G.; Smith, M.; Farmer, B.; Williams, P.E.; Coate, K.C.; Printz, R.L.; O’Brien, R.M.; Cherrington, A.D. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight 2017, 2, e91863. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [Green Version]
- Marciano, L.; Camerini, A.-L.; Schulz, P.J. The Role of Health Literacy in Diabetes Knowledge, Self-Care, and Glycemic Control: A Meta-analysis. J. Gen. Intern. Med. 2019, 34, 1007–1017. [Google Scholar] [CrossRef]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care 2015, 3, 000093. [Google Scholar] [CrossRef]
- Roglic, G.; Unwin, N.; Bennett, P.H.; Mathers, C.; Tuomilehto, J.; Nag, S.; Connolly, V.; King, H. The burden of mortality attributable to diabetes: Realistic estimates for the year 2000. Diabetes Care 2005, 28, 2130–2135. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Tancredi, M.; Kosiborod, M.; Dahlqvist, S.; Rosengren, A.; Svensson, A.-M.; Pivodic, A.; Gudbjörnsdóttir, S.; Wedel, H.; Clements, M.; Lind, M. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef] [Green Version]
- Diabetes UK. Diabetes in the UK 2010: Key Statistics on Diabetes; Diabetes UK: London, UK, 2010. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; pp. 9–44. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of Diabetes among Men and Women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef]
- Marine, N.; Vinik, A.I.; Edelstein, I.; Jackson, W.P.U. Diabetes, Hyperglycemia and Glycosuria among Indians, Malays and Africans (Bantu) in Cape Town, South Africa. Diabetes 1969, 18, 840–857. [Google Scholar] [CrossRef]
- Goldberg, M.D.; Marine, N.; Ribeiro, F.; Campbell, G.D.; Vinik, A.I.; Jackson, W.P. Prevalence of glycosuria and diabetes among Indians and Bantu. S. Afr. Med. J. 1969, 43, 733–738. [Google Scholar]
- Jackson, W. Epidemiology of Diabetes in South Africa. In Advances in Metabolic Disorders; Elsevier BV: Amsterdam, The Netherlands, 1978; Volume 9, pp. 111–146. [Google Scholar]
- Cassidy, J.T. Diabetes in Fiji. N. Z. Med. J. 1967, 66, 167–172. [Google Scholar]
- Zimmet, P.; Taylor, R.; Ram, P.; King, H.; Sloman, G.; Raper, L.R.; Hunt, D. Prevalence of diabetes and impaired glucose tolerance in the biracial (melanesian and indian) population of fiji: A rural-urban comparison. Am. J. Epidemiol. 1983, 118, 673–688. [Google Scholar] [CrossRef]
- Poon-King, T.; Henry, M.; Rampersad, F. Prevalence and natural history of diabetes in trinidad. Lancet 1968, 291, 155–160. [Google Scholar] [CrossRef]
- Sellu, D.P.; Lynn, J.A. The Southall diabetes survey: Prevalence of known diabetes in Asians and Europeans. BMJ 1985, 291, 1347–1348. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Shetty, A.S.; Nanditha, A. Trends in prevalence of diabetes in Asian countries. World J. Diabetes 2012, 3, 110–117. [Google Scholar] [CrossRef]
- Mohanty, S.A.; Woolhandler, S.; Himmelstein, D.U.; Bor, D.H. Diabetes and Cardiovascular Disease Among Asian Indians in the United States. J. Gen. Intern. Med. 2005, 20, 474–478. [Google Scholar] [CrossRef]
- Gupta, L.S.; Wu, C.C.; Young, S.; Perlman, S.E. Prevalence of diabetes in New York City, 2002–2008: Comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care 2011, 34, 1791–1793. [Google Scholar] [CrossRef]
- Sicree, R. The Global Burden of Diabetes. Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2003. [Google Scholar]
- Guariguata, L.; Whiting, D.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- International Diabetes Federation. Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2006. [Google Scholar]
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.H.; Hu, F.B. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Cho, N.H.; Tajima, N.; Shaw, J. Diabetes in the Western Pacific Region—Past, Present and Future. Diabetes Res. Clin. Pract. 2014, 103, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Ma, R.C.W. Diabetes in South-East Asia: An update. Diabetes Res. Clin. Pract. 2014, 103, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.P.; Alkema, L.; Tai, E.S.; Tan, K.H.X.; Yang, Q.; Lim, W.-Y.; Teo, Y.Y.; Cheng, C.-Y.; Wang, X.; Wong, T.Y.; et al. Forecasting the burden of type 2 diabetes in Singapore using a demographic epidemiological model of Singapore. BMJ Open Diabetes Res. Care 2014, 2, e000012. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosing Diabetes and Learning about Prediabetes. Available online: http://www.diabetes.org/diabetes-basics/diagnosis/ (accessed on 3 March 2019).
- Taylor, R.; Zimmet, P. Limitation of Fasting Plasma Glucose for the Diagnosis of Diabetes Mellitus. Diabetes Care 1981, 4, 556–558. [Google Scholar] [CrossRef]
- Bonora, E.; Tuomilehto, J. The Pros and Cons of Diagnosing Diabetes with A1C. Diabetes Care 2011, 34, S184–S190. [Google Scholar] [CrossRef]
- Kim, D.-L.; Kim, S.D.; Kim, S.K.; Park, S.; Song, K.H. Is an oral glucose tolerance test still valid for diagnosing diabetes mellitus? Diabetes Metab. J. 2016, 40, 118–128. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Cerasi, E.; Luft, R.; Efendic, S. Decreased Sensitivity of the Pancreatic Beta Cells to Glucose in Prediabetic and Diabetic Subjects: A Glucose Dose-Response Study. Diabetes 1972, 21, 224–234. [Google Scholar] [CrossRef]
- Gossain, V.V.; Aldasouqi, S. The challenge of undiagnosed pre-diabetes, diabetes and associated cardiovascular disease. Int. J. Diabetes Mellit. 2010, 2, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.W.Y.; Sequeira, I.R.; Plank, L.D.; Poppitt, S.D. Prevalence of Pre-Diabetes across Ethnicities: A Review of Impaired Fasting Glucose (IFG) and Impaired Glucose Tolerance (IGT) for Classification of Dysglycaemia. Nutrients 2017, 9, 1273. [Google Scholar] [CrossRef]
- Tuso, P. Prediabetes and Lifestyle Modification: Time to Prevent a Preventable Disease. Perm. J. 2014, 18, 88–93. [Google Scholar] [CrossRef]
- Shen, J.; Kondal, D.; Rubinstein, A.; Irazola, V.; Gutiérrez, L.; Miranda, J.J.; Bernabé-Ortiz, A.; Lazo-Porras, M.; Levitt, N.; Steyn, K.; et al. A Multiethnic Study of Pre-Diabetes and Diabetes in LMIC. Glob. Heart 2016, 11, 61–70. [Google Scholar] [CrossRef]
- Alanazi, N.H.; Alsharif, M.M.; Rasool, G.; Alruwaili, A.B.H.; Alrowaili, A.M.Z.; Aldaghmi, A.S.; Al Shkra, M.K.D.; Alrasheedi, F.A.; Alenezi, G.S.; Alanazi, M.T. Prevalence of diabetes and its relation with age and sex in Turaif city, northern Saudi Arabia in 2016–2017. Electron. Physician 2017, 9, 5294–5297. [Google Scholar] [CrossRef]
- News Release: FDA Approves Farxiga to Treat Type 2 Diabetes. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-pediatric-patients-type-2-diabetes (accessed on 10 December 2018).
- Papaetis, G.S. Incretin-based therapies in prediabetes: Current evidence and future perspectives. World J. Diabetes 2014, 5, 817–834. [Google Scholar] [CrossRef]
- Runge, C.F. Economic consequences of the obese. Diabetes 2007, 56, 2668–2672. [Google Scholar] [CrossRef]
- Garber, A.J. Obesity and type 2 diabetes: Which patients are at risk? Diabetes Obes. Metab. 2012, 14, 399–408. [Google Scholar] [CrossRef]
- Barbarroja, N.; López-Pedrera, R.; Mayas, M.D.; García-Fuentes, E.; Garrido-Sánchez, L.; Macías-González, M.; El Bekay, R.; Vidal-Puig, A.; Tinahones, F.J. The obese healthy paradox: Is inflammation the answer? Biochem. J. 2010, 430, 141–149. [Google Scholar] [CrossRef]
- Purkayastha, S.; Zhang, G.; Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 2011, 17, 883. [Google Scholar] [CrossRef]
- Wild, S.H.; Byrne, C.D. ABC of obesity. Risk factors for diabetes and coronary heart disease. BMJ 2006, 333, 1009–1011. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J.H.; Davies, M. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Balkau, B.; Mhamdi, L.; Oppert, J.M.; Nolan, J.; Golay, A.; Porcellati, F.; Laakso, M.; Ferrannini, E.; EGIR-RISC Study Group. Physical activity and insulin sensitivity: The RISC study. Diabetes 2008, 57, 2613–2618. [Google Scholar] [CrossRef]
- Yates, T.; Henson, J.; Edwardson, C.; Dunstan, D.; Bodicoat, D.H.; Khunti, K.; Davies, M.J. Objectively measured sedentary time and associations with insulin sensitivity: Importance of reallocating sedentary time to physical activity. Prev. Med. 2015, 76, 79–83. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention, U.S. Dept of Health and Human Service: Atlanta, GA, USA, 2017.
- Ali, A. Health Survey for England 2006: Volume 1 Cardiovascular Disease and Risk Factors in Adults; National Centre for Social Research: London, UK, 2008. [Google Scholar]
- Suastika, K.; Dwipayana, P.; Saraswati, I.M.R.; Kuswardhani, T.; Astika, N.; Putrawan, I.B.; Matsumoto, K.; Kajiwara, N.; Taniguchi, H. Relationship between age and metabolic disorders in the population of Bali. J. Clin. Gerontol. Geriatr. 2011, 2, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.-N.; Lin, M.-H.; Lai, H.-Y.; Hwang, S.-J.; Chen, L.-K.; Chiou, S.-T. Risk factors of new onset diabetes mellitus among elderly Chinese in rural Taiwan. Age Ageing 2009, 39, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.M.; Halter, J.B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E7–E12. [Google Scholar] [CrossRef] [Green Version]
- Garawi, F.; Devries, K.; Thorogood, N.; Uauy, R. Global differences between women and men in the prevalence of obesity: Is there an association with gender inequality? Eur. J. Clin. Nutr. 2014, 68, 1101–1106. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.M. Lipid metabolism in women. Proc. Nutr. Soc. 2004, 63, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Krishnaswami, S.; Harris, T.B.; Katsiaras, A.; Kritchevsky, S.B.; Simonsick, E.M.; Nevitt, M.; Holvoet, P.; Newman, A.B. Obesity, Regional Body Fat Distribution, and the Metabolic Syndrome in Older Men and Women. Arch. Intern. Med. 2005, 165, 777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Xu, M.; Gu, W.; Xi, Y.; Qi, L.; Li, B.; Wang, W. Brown Adipose Tissue Activation Is Inversely Related to Central Obesity and Metabolic Parameters in Adult Human. PLoS ONE 2015, 10, e0123795. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiebinger, L.; Klinge, I.; Paik, H.Y.; Sánchez de Madariaga, I.; Schraudner, M.; Stefanick, M. Gendered Innovations in Science, Health & Medicine, Engineering, and Environment, 2011–2018. Available online: http://genderedinnovations.stanford.edu/ (accessed on 10 January 2015).
- EUGenMed, Cardiovascular Clinical Study Group; Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2015, 37, 24–34. [Google Scholar]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [Green Version]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Cheung, B.M.Y.; Li, C. Diabetes and Hypertension: Is There a Common Metabolic Pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Cheung, B.M.Y. The Hypertension–Diabetes Continuum. J. Cardiovasc. Pharmacol. 2010, 55, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Midthjell, K.; Grill, V. Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: An 11-year follow-up of incidence of diabetes in the Nord-Trøndelag study. Diabetologia 2004, 47, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Willi, C.; Bodenmann, P.; Ghali, W.A.; Faris, P.D.; Cornuz, J. Active Smoking and the Risk of Type 2 DiabetesA Systematic Review and Meta-analysis. JAMA 2007, 298, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, B. Cigarette smoking and diabetes. Prog. Cardiovasc. Dis. 2003, 45, 405–413. [Google Scholar] [CrossRef]
- Facchini, F.; Hollenbeck, C.; Jeppesen, J.; Chen, Y.-D.I.; Reaven, G. Insulin resistance and cigarette smoking. Lancet 1992, 339, 1128–1130. [Google Scholar] [CrossRef]
- Madsbad, S.; McNair, P.; Christensen, M.S.; Christiansen, C.; Faber, O.K.; Binder, C.; Transbol, I. Influence of Smoking on Insulin Requirement and Metbolic Status in Diabetes Mellitus. Diabetes Care 1980, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Epifano, L.; Di Vincenzo, A.; Fanelli, C.; Porcellati, E.; Perriello, G.; De Feo, P.; Motolese, M.; Brunetti, P.; Bolli, G.B.; Vincenzo, A.; et al. Effect of cigarette smoking and of a transdermal nicotine delivery system on glucoregulation in type 2 diabetes mellitus. Eur. J. Clin. Pharmacol. 1992, 43, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.C.; Iniestra, F.; Ariza, C.R. Acute Effect of Cigarette Smoking on Glucose Tolerance and Other Cardiovascular Risk Factors. Diabetes Care 1996, 19, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Perreault, L.; Hunerdosse, D.M.; Koehler, M.C.; Samek, A.M.; Eckel, R.H. Intramuscular Lipid Metabolism in the Insulin Resistance of Smoking. Diabetes 2009, 58, 2220–2227. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Permert, J.; Håkansson, N.; Näslund, I.; Bergkvist, L.; Wolk, A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br. J. Cancer 2005, 93, 1310. [Google Scholar] [CrossRef]
- Yun, J.E.; Kimm, H.; Choi, Y.J.; Jee, S.H.; Huh, K.B. Smoking Is Associated with Abdominal Obesity, Not Overall Obesity, in Men with Type 2 Diabetes. J. Prev. Med. Public Health 2012, 45, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, A.; Miura, K.; Kadowaki, S.; Azuma, K.; Tanaka, S.; Hisamatsu, T.; Arima, H.; Kadota, A.; Miyagawa, N.; Takashima, N.; et al. Lifetime cigarette smoking is associated with abdominal obesity in a community-based sample of Japanese men: The Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA). Prev. Med. Rep. 2016, 4, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, H.; Hellstrom-Lindahl, E.; Grill, V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism 2005, 54, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Petre, M.A.; Lehman, M.A.; Raha, S.; Gerstein, H.C.; Morrison, K.M.; Holloway, A.C. Maternal nicotine exposure increases oxidative stress in the offspring. Free. Radic. Boil. Med. 2008, 44, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Hur, N.W.; Kim, H.C.; Nam, C.M.; Jee, S.H.; Lee, H.C.; Suh, I. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Li, T.-C.; Chang, P.-C.; Liu, C.-S.; Lin, W.-Y.; Wu, M.-T.; Li, C.-I.; Lai, M.-M.; Lin, C.-C. Association among cigarette smoking, metabolic syndrome, and its individual components: The metabolic syndrome study in Taiwan. Metabolism 2008, 57, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.T.C.; Chan, J.; Tsang, L.; Critchley, J.; Cockram, C. Smoking and diabetes in Chinese men. Postgrad. Med. J. 2001, 77, 240–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.O. Teenage smoking in China. J. Adolesc. 1999, 22, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.O. Smoking in China. BMJ 1995, 310. [Google Scholar] [CrossRef]
- Knott, C.; Bell, S.; Britton, A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals from 38 Observational Studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef]
- Doria, A.; Patti, M.-E.; Kahn, C.R. The Emerging Genetic Architecture of Type 2 Diabetes. Cell Metab. 2008, 8, 186–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.S. The Search for Genetic Risk Factors of Type 2 Diabetes Mellitus. Diabetes Metab. J. 2011, 35, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.; Oda, N.; Cox, N.J.; Li, X.; Orho-Melander, M.; Hara, M.; Hinokio, Y.; Lindner, T.H.; Mashima, H.; Schwarz, P.E.; et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat. Genet. 2000, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Vionnet, N.; Hani, E.H.; Dupont, S.; Gallina, S.; Francke, S.; Dotte, S.; De Matos, F.; Durand, E.; Leprêtre, F.; Lecoeur, C.; et al. Genomewide Search for Type 2 Diabetes-Susceptibility Genes in French Whites: Evidence for a Novel Susceptibility Locus for Early-Onset Diabetes on Chromosome 3q27-qter and Independent Replication of a Type 2–Diabetes Locus on Chromosome 1q21–q24. Am. J. Hum. Genet. 2000, 67, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, D.; Hirschhorn, J.N.; Klannemark, M.; Lindgren, C.M.; Vohl, M.C.; Nemesh, J.; Lane, C.R.; Schaffner, S.F.; Bolk, S.; Brewer, C.; et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet. 2000, 26, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Weedon, M.N.; Owen, K.R.; Turner, M.J.; Knight, B.A.; Hitman, G.; Walker, M.; Levy, J.C.; Sampson, M.; Halford, S.; et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003, 52, 568–572. [Google Scholar] [CrossRef]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef]
- Kim, H.-I.; Ahn, Y.-H. Role of peroxisome proliferator-activated receptor-γ in the glucose-sensing apparatus of liver and β-cells. Diabetes 2004, 53 (Suppl. 1), S60–S65. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, M.; Cui, T.; Xiong, C.; Xu, K.; Zhong, W.; Xiao, Y.; Floyd, D.; Liang, J.; Li, E.; et al. Selective disruption of PPARγ2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2004, 101, 10703–10708. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Rangwala, S.M.; Lazar, M.A. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol. Sci. 2004, 25, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Haghvirdizadeh, P.; Mohamed, Z.; Abdullah, N.A.; Haghvirdizadeh, P.; Haerian, M.S.; Haerian, B.S. KCNJ11: Genetic Polymorphisms and Risk of Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gloyn, A.L.; Pearson, E.R.; Antcliff, J.F.; Proks, P.; Bruining, G.J.; Slingerland, A.S.; Silva, J.M.; Molnes, J.; Edghill, E.L.; Mackay, D.; et al. Activating Mutations in the Gene Encoding the ATP-Sensitive Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes. N. Engl. J. Med. 2004, 350, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Florez, J.C.; Burtt, N.; De Bakker, P.I.; Almgren, P.; Tuomi, T.; Holmkvist, J.; Gaudet, D.; Hudson, T.J.; Schaffner, S.F.; Daly, M.J.; et al. Haplotype structure and genotype-phenotype correlations of the sulfonylurea receptor and the islet ATP-sensitive potassium channel gene region. Diabetes 2004, 53, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.-M.D.; Hansen, L.; Carstensen, B.; Echwald, S.M.; Drivsholm, T.; Glümer, C.; Thorsteinsson, B.; Borch-Johnsen, K.; Hansen, T.; Pedersen, O. The E23K Variant of Kir6.2 Associates with Impaired Post-OGTT Serum Insulin Response and Increased Risk of Type 2 Diabetes. Diabetes 2003, 52, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Zeggini, E.; Weedon, M.N.; Lindgren, C.M.; Frayling, T.M.; Elliott, K.S.; Lango, H.; Timpson, N.J.; Perry, J.R.; Rayner, N.W.; Freathy, R.M.; et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007, 316, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Voight, B.F.; Lyssenko, V.; Burtt, N.P.; De Bakker, P.I.W.; Chen, H.; Roix, J.J.; Kathiresan, S.; Hirschhorn, J.N.; Daly, M.J.; et al. Genome-Wide Association Analysis Identifies Loci for Type 2 Diabetes and Triglyceride Levels. Science 2007, 316, 1331–1336. [Google Scholar]
- Steinthorsdottir, V.; Thorleifsson, G.; Reynisdóttir, I.; Benediktsson, R.; Jonsdottir, T.; Walters, G.B.; Styrkarsdottir, U.; Gretarsdottir, S.; Emilsson, V.; Ghosh, S.; et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat. Genet. 2007, 39, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Bélisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Billings, L.K.; Florez, J.C. The genetics of type 2 diabetes: What have we learned from GWAS? Ann. N. Y. Acad. Sci. 2010, 1212, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Mukherjee, J.J.; Ramachandran, A.; Saboo, B.; Shaikh, S.; Venkataraman, S.; Bantwal, G.; Das, A.K. Hypoglycemia: The neglected complication. Indian J. Endocrinol. Metab. 2013, 17, 819–834. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Self-Monitoring of Blood Glucose. Clin. Diabetes 2002, 20, 48. [Google Scholar] [CrossRef]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S55–S64. [Google Scholar] [CrossRef] [PubMed]
- Erbach, M.; Freckmann, G.; Hinzmann, R.; Kulzer, B.; Ziegler, R.; Heinemann, L.; Schnell, O. Interferences and Limitations in Blood Glucose Self-Testing: An Overview of the Current Knowledge. J. Diabetes Sci. Technol. 2016, 10, 1161–1168. [Google Scholar] [CrossRef]
- Heinemann, L. Finger Pricking and Pain: A Never Ending Story. J. Diabetes Sci. Technol. 2008, 2, 919–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef]
- Coyle, S.; Curto, V.F.; Benito-Lopez, F.; Florea, L.; Diamond, D. Chapter 2.1—Wearable Bio and Chemical Sensors. In Wearable Sensors; Sazonov, E., Neuman, M.R., Eds.; Academic Press: Oxford, UK, 2014; pp. 65–83. [Google Scholar]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef]
- Mathew, T.L.; Pownraj, P.; Abdulla, S.; Pullithadathil, B. Technologies for Clinical Diagnosis Using Expired Human Breath Analysis. Diagnostics 2015, 5, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandodkar, A.J.; You, J.-M.; Kim, N.-H.; Gu, Y.; Kumar, R.; Mohan, A.M.V.; Kurniawan, J.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-invasive Analyte Access and Sensing through Eccrine Sweat: Challenges and Outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Yamada, K.; Ohishi, K.; Gilbert, A.; Akasaka, M.; Yoshida, N.; Yoshimura, R. Measurement of natural carbon isotopic composition of acetone in human urine. Anal. Bioanal. Chem. 2016, 408, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Lilly lecture 1987. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988, 37, 667–687. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, M.F.; Knowler, W.C.; Pettitt, D.J.; Nelson, R.G.; Mott, D.M.; Bennett, P.H. The Natural History of Impaired Glucose Tolerance in the Pima Indians. N. Engl. J. Med. 1988, 319, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; DeFronzo, R.A.; Abdul-Ghani, M.A. Treatment of prediabetes. World J. Diabetes 2015, 6, 1207–1222. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Knowler, W.C.; Hamman, R.F.; Edelstein, S.L.; Barrett-Connor, E.; Ehrmann, D.A.; Walker, E.A.; Fowler, S.E.; Nathan, D.M.; Kahn, S.E. Prevention of Type 2 Diabetes with Troglitazone in the Diabetes Prevention Program. Diabetes 2005, 54, 1150–1156. [Google Scholar] [PubMed]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [PubMed]
- DeFronzo, R.A.; Tripathy, D.; Schwenke, D.C.; Banerji, M.; Bray, G.A.; Buchanan, T.A.; Clement, S.C.; Henry, R.R.; Hodis, H.N.; Kitabchi, A.E.; et al. Pioglitazone for Diabetes Prevention in Impaired Glucose Tolerance. N. Engl. J. Med. 2011, 364, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- Slentz, C.A.; Tanner, C.J.; Bateman, L.A.; Durheim, M.T.; Huffman, K.M.; Houmard, J.A.; Kraus, W.E. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 2009, 32, 1807–1811. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, P.R.; Kahn, B.B. Glucose Transporters and Insulin Action — Implications for Insulin Resistance and Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 248–257. [Google Scholar] [CrossRef]
- Chen, Z.; Black, M.H.; Watanabe, R.M.; Trigo, E.; Takayanagi, M.; Lawrence, J.M.; Buchanan, T.A.; Xiang, A.H. Self-reported physical activity is associated with beta-cell function in Mexican American adults. Diabetes Care 2013, 36, 638–644. [Google Scholar] [CrossRef]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2015, 12, 4–20. [Google Scholar] [CrossRef]
- Malin, S.K.; Gerber, R.; Chipkin, S.R.; Braun, B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 2012, 35, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Qi, L.; Fahey, G.C.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Kerrison, G.; Gillis, R.B.; Jiwani, S.I.; Alzahrani, Q.; Kok, S.; Harding, S.E.; Shaw, I.; Adams, G.G. The Effectiveness of Lifestyle Adaptation for the Prevention of Prediabetes in Adults: A Systematic Review. J. Diabetes Res. 2017, 2017, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of Diet and Exercise in Preventing NIDDM in People With Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss with Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; (Idpp), I.D.P.P. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Kitabchi, A.E.; Temprosa, M.; Knowler, W.C.; Kahn, S.E.; Fowler, S.E.; Haffner, S.M.; Andres, R.; Saudek, C.; Edelstein, S.L.; Arakaki, R.; et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes 2005, 54, 2404–2414. [Google Scholar]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef]
- Saito, T.; Nishida, J.; Izumi, T.; Omura, M.; Takagi, T.; Fukunaga, R.; Bandai, Y.; Tajima, N.; Nakamura, Y.; for the Zensharen Study for Prevention of Lifestyle Diseases Group; et al. Lifestyle Modification and Prevention of Type 2 Diabetes in Overweight Japanese with Impaired Fasting Glucose Levels: A Randomized Controlled Trial. Arch. Intern. Med. 2011, 171, 1352. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Goldstein, M.G.; Acton, K.J.; Birch, L.L.; Jakicic, J.M.; Sallis, J.F.; Smith-West, D.; Jeffery, R.W.; Surwit, R.S. Behavioral Science Research in Diabetes: Lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care 2001, 24, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venditti, E.M.; A. Bray, G.; Carrion-Petersen, M.L.; Delahanty, L.M.; Edelstein, S.L.; Hamman, R.F.; Hoskin, M.A.; Knowler, W.C.; Ma, Y. First versus repeat treatment with a lifestyle intervention program: Attendance and weight loss outcomes. Int. J. Obes. 2008, 32, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K.; Battjes-Fries, M.C.; Patijn, O.N.; Pijl, H.; Witkamp, R.F.; De Visser, M.; Van Der Zijl, N.; De Vries, M.; Voshol, P.J. Nutrition and lifestyle intervention in type 2 diabetes: Pilot study in the Netherlands showing improved glucose control and reduction in glucose lowering medication. BMJ Nutr. Prev. Health 2019. [Google Scholar] [CrossRef]
- Ministry of Health and Care Services. Care Guidelines and Programmes for Persons with Pre-Diabetes; Ministry of Health and Care Services: Oslo, Norway, 2017.
- Song, R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care 2016, 39, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wondisford, F.E. Metformin Action: Concentrations Matter. Cell Metab. 2015, 21, 159–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gormsen, L.C.; Sundelin, E.I.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Christensen, M.M.H.; Brøsen, K.; Frøkiær, J.; Jessen, N. In Vivo Imaging of Human 11C-Metformin in Peripheral Organs: Dosimetry, Biodistribution and Kinetic Analyses. J. Nucl. Med. 2016, 57, 1920–1926. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.M.; DeFronzo, R.A. Efficacy of Metformin in Patients with Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar]
- DeFronzo, R.A.; Barzilai, N.; Simonson, D.C. Mechanism of Metformin Action in Obese and Lean Noninsulin-Dependent Diabetic Subjects. J. Clin. Endocrinol. Metab. 1991, 73, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1996, 81, 4059–4067. [Google Scholar] [PubMed]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R.; UK Prospective Diabetes Study (UKPDS) Group. Glycemic Control with Diet, Sulfonylurea, Metformin, or Insulin in Patients With Type 2 Diabetes MellitusProgressive Requirement for Multiple Therapies (UKPDS 49). JAMA 1999, 281, 2005. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Webster, J.; Sum, C.-F.; Heseltine, L.; Argyraki, M.; Cooper, B.; Taylor, R. The impact of metformin therapy on hepatic glucose production and skeletal muscle glycogen synthase activity in overweight type II diabetic patients. Metabolism 1993, 42, 1217–1222. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- El-Mir, M.-Y.; Nogueira, V.; Fontaine, E.; Averet, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Boil. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Ross, F.A.; Mackintosh, C.; Hardie, D.G. AMP-activated protein kinase: A cellular energy sensor that comes in 12 flavours. FEBS J. 2016, 283, 2987–3001. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK - a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Boil. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Hebrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Thazhath, S.S.; Bound, M.J.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: Stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes Res. Clin. Pract. 2014, 106, e3–e6. [Google Scholar] [CrossRef] [PubMed]
- Thondam, S.K.; Cross, A.; Cuthbertson, D.; Wilding, J.P.; Daousi, C. Effects of chronic treatment with metformin on dipeptidyl peptidase-4 activity, glucagon-like peptide 1 and ghrelin in obese patients with Type 2 diabetes mellitus. Diabet. Med. 2012, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, J.; Patterson, S.; O’Harte, F.P.; Bell, P.M. Addition of metformin to exogenous glucagon-like peptide–1 results in increased serum glucagon-like peptide–1 concentrations and greater glucose lowering in type 2 diabetes mellitus. Metabolism 2011, 60, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin Increases AMP-Activated Protein Kinase Activity in Skeletal Muscle of Subjects with Type 2 Diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. Impaired Fasting Glucose and Impaired Glucose Tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Phua, W.W.T.; Wong, M.X.Y.; Liao, Z.; Tan, N.S. An aPPARent Functional Consequence in Skeletal Muscle Physiology via Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2018, 19, 1425. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Spollett, G.R.; Page, S.L.; Rife, F.S.; Walton, V.; Shulman, G.I.; Maggs, D.G. Efficacy and Metabolic Effects of Metformin and Troglitazone in Type II Diabetes Mellitus. N. Engl. J. Med. 1998, 338, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Mahankali, A.; Matsuda, M.; Glass, L.; Mahankali, S.; Ferrannini, E.; Cusi, K.; Mandarino, L.J.; DeFronzo, R.A. Improved Glycemic Control and Enhanced Insulin Sensitivity in Type 2 Diabetic Subjects Treated with Pioglitazone. Diabetes Care 2001, 24, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; He, H.; Mandarino, L.J.; DeFronzo, R.A. Rosiglitazone Improves Downstream Insulin Receptor Signaling in Type 2 Diabetic Patients. Diabetes 2003, 52, 1943–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkowitz, K.; Peters, R.; Kjos, S.L.; Goico, J.; Marroquin, A.; Dunn, M.E.; Xiang, A.; Azen, S.; Buchanan, T.A. Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM. Diabetes 1996, 45, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.H.; Peters, R.K.; Kjos, S.L.; Marroquin, A.; Goico, J.; Ochoa, C.; Kawakubo, M.; Buchanan, T.A. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006, 55, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H.; Peters, R.K.; Kjos, S.L.; Marroquin, A.; Goico, J.; Ochoa, C.; Tan, S.; Berkowitz, K.; Hodis, H.N.; et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002, 51, 2796–2803. [Google Scholar] [CrossRef]
- Bischoff, H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin. Investig. Med. 1995, 18, 303–311. [Google Scholar]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, R.; Tajima, N.; Iwamoto, Y.; Kashiwagi, A.; Shimamoto, K.; Kaku, K. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009, 373, 1607–1614. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tanaka, A.; Sata, M.; Dai, K.; Shibata, Y.; Inoue, Y.; Ikenaga, H.; Kishimoto, S.; Ogasawara, K.; Takashima, A.; et al. α-Glucosidase inhibitor miglitol attenuates glucose fluctuation, heart rate variability and sympathetic activity in patients with type 2 diabetes and acute coronary syndrome: A multicenter randomized controlled (MACS) study. Cardiovasc. Diabetol. 2017, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Buse, J.B.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Wintle, M.E.; Maggs, D.G. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 2008, 24, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Rossner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of the European Public Assessment Report (EPAR) for Forxiga; European Medicines Agency: London, UK, 2012.
- FDA News Release: FDA Approves Jardiance to Treat Type 2 Diabetes. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-jardiance-reduce-cardiovascular-death-adults-type-2-diabetes (accessed on 10 December 2018).
- American Diabetes Association. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. Clin. Diabetes 2015, 33, 97–111. [Google Scholar] [CrossRef]
- Hemmingsen, B.; Krogh, J.; Metzendorf, M.I.; Richter, B. Sodium-glucose cotransporter (SGLT) 2 inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2016, 4, CD012106. [Google Scholar]
- Zinman, B.; Wanner, C.; Hantel, S.; Mattheus, M.; Devins, T.; Broedl, U.C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Squibb, B.-M. US Food and Drug Administration Endocrinologic & Metabolic Advisory Committee Background Document: Dapagliflozin, BMS-512148, NDA 202293; Food and Drug Administration: Princeton, NJ, USA, 2011.
- Mather, A.; Pollock, C. Glucose handling by the kidney. Kidney Int. 2011, 79, S1–S6. [Google Scholar] [CrossRef] [Green Version]
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005, 54, 3427–3434. [Google Scholar] [CrossRef]
- List, J.F.; Whaley, J.M. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011, 79, S20–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrom, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Pories, W.J.; MacDonald, K.G., Jr.; Flickinger, E.G.; Dohm, G.L.; Sinha, M.K.; Barakat, H.A.; May, H.J.; Khazanie, P.; Swanson, M.S.; Morgan, E.; et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann. Surg. 1992, 215, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts in tumor microenvironment – Accomplices in tumor malignancy. Cell. Immunol. 2018. [Google Scholar] [CrossRef]
- Biadgo, B.; Abebe, M. Type 2 Diabetes Mellitus and Its Association with the Risk of Pancreatic Carcinogenesis: A Review. Korean J. Gastroenterol. 2016, 67, 168–177. [Google Scholar] [CrossRef]
- Magruder, J.T.; Elahi, D.; Andersen, D.K. Diabetes and Pancreatic Cancer: Chicken or Egg? Pancreas 2011, 40, 339–351. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A Consensus Report. CA A Cancer J. Clin. 2010, 60, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Bonovas, S.; Filioussi, K.; Tsantes, A. Diabetes mellitus and risk of prostate cancer: A meta-analysis. Diabetologia 2004, 47, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.S.; Giovannucci, E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.L.; Sanderson, M.; Zheng, W.; Fadden, M.K. Diabetes mellitus and prostate cancer risk among older men: Population-based case–control study. Br. J. Cancer 2004, 90, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The Prevalence of Comorbid Depression in Adults with Diabetes. Diabetes Care 2001, 24, 1069. [Google Scholar] [CrossRef]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and Type 2 Diabetes Over the Lifespan. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Monte, S.M.; Wands, J.R. Alzheimer’s Disease is Type 3 Diabetes—Evidence Reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.M.; Flyvbjerg, A. Prevention and early detection of vascular complications of diabetes. BMJ 2006, 333, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L., III; Klein, R.; American Diabetes Association. Retinopathy in Diabetes. Diabetes Care 2004, 27 (Suppl. 1), s84–s87. [Google Scholar] [CrossRef] [Green Version]
- Jeganathan, V.S.E.; Wang, J.J.; Wong, T.Y. Ocular Associations of Diabetes Other Than Diabetic Retinopathy. Diabetes Care 2008, 31, 1905–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandare, N.N.; Keny, M.S.; Nevrekar, R.P.; Bhandare, P.N. Diabetic Tongue—Could it be a Diagnostic Criterion? J. Fam. Med. Prim. Care 2014, 3, 290–291. [Google Scholar]
- Matheus, A.S.; Tannus, L.R.; Cobas, R.A.; Palma, C.C.; Negrato, C.A.; Gomes, M.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Nazar, C.M.J. Diabetic nephropathy; principles of diagnosis and treatment of diabetic kidney disease. J. Nephropharmacol. 2014, 3, 15–20. [Google Scholar]
- Aldukhayel, A. Prevalence of diabetic nephropathy among Type 2 diabetic patients in some of the Arab countries. Int. J. Health Sci. 2017, 11, 1–4. [Google Scholar]
- American Diabetes Association. Nephropathy in Diabetes. Diabetes Care 2004, 27 (Suppl. 1), s79–s83. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.K. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renov. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Bellastella, G.; Esposito, K. Diabetes and sexual dysfunction: Current perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 95–105. [Google Scholar]
- Bortolotti, A.; Colli, E.; Lavezzari, M.; Landoni, M.; Fedele, D.; Coscelli, C.; Santeusanio, F.; Chatenoud, L.; Parazzini, F. Erectile dysfunction in Type 1 and Type 2 diabetics in Italy. Int. J. Epidemiol. 2000, 29, 524–531. [Google Scholar] [Green Version]
- Lue, T.F. Sexual Dysfunction in Diabetes; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Várkonyi, T.; Kempler, P. Chapter 16–Sexual Dysfunction in Diabetes. In Handbook of Clinical Neurology; Zochodne, D.W., Malik, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 126, pp. 223–232. [Google Scholar]
- Ahmed, M.R.; Shaaban, M.M.; Sedik, W.F.; Mohamed, T.Y. Prevalence and differences between type 1 and type 2 diabetes mellitus regarding female sexual dysfunction: A cross-sectional Egyptian study. J. Psychosom. Obstet. Gynaecol. 2018, 39, 176–181. [Google Scholar] [CrossRef] [PubMed]
- De Macedo, G.M.C.; Nunes, S.; Barreto, T. Skin disorders in diabetes mellitus: An epidemiology and physiopathology review. Diabetol. Metab. Syndr. 2016, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Duff, M.; Demidova, O.; Blackburn, S.; Shubrook, J. Cutaneous Manifestations of Diabetes Mellitus. Clin. Diabetes 2015, 33, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, A.K.; Nones, C.F.; Reis, R.C.; Chichorro, J.G.; Cunha, J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Juster-Switlyk, K.; Smith, A.G. Updates in diabetic peripheral neuropathy. F1000Research 2016, 5, 738. [Google Scholar] [CrossRef]
- Román-Pintos, L.M.; Villegas-Rivera, G.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. Diabetic Polyneuropathy in Type 2 Diabetes Mellitus: Inflammation, Oxidative Stress, and Mitochondrial Function. J. Diabetes Res. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
| FPG | PG in OGTT | A1C | |
|---|---|---|---|
| Normal | 100 mg/dL or 5.5 mmol/L | 140 mg/dL or 7.8 mmol/L | 5.7% or 39 mmol/mol |
| Pre-Diabetes | 100 mg/dL or 5.5 mmol/L | 140 mg/dL or 7.8 mmol/L | 5.7% or 39 mmol/mol |
| Diabetes | 126 mg/dL or 7.0 mmol/L | 200 mg/dL or 11.1 mmol/L | 6.5% or 48 mmol/mol |
| Year of Genome Wide Significance (GWS) | Locus | Marker | Chr | Type of Mutation | Encoded Protein of Wild Type | Trait |
|---|---|---|---|---|---|---|
| 2000 | PPARG | rs1801282 [96] | 3 | Missense: Pro12Ala | PPAR-γ a | T2DM |
| 2003 | KCNJ11 | rs5219 [97] | 11 | Missense: Glu23Lys | Kir6.2 of pancreatic β-cells a | T2DM |
| 2007 | CDKAL1 | rs7754840 [107,108] | 6 | Intronic | CDK5 regulatory subunit-associated protein 1-like 1 [109] | T2DM |
| SLC30A8 | rs13266634 [110,111] | 8 | Missense: Arg325Trp | Islet-specific zinc membrane transporter (ZnT8) a | T2DM and FG | |
| IGF2BP2 | rs4402960 [107,108] | 3 | Intronic | Insulin-like growth factor 2 mRNA-binding protein a | T2DM | |
| CDKN2A/B | rs10811661 [107,108] | 9 | 25 kb upstream | p16 (INK4A) a | T2DM |
| Direct Causes | Known Links | Possible Links |
|---|---|---|
| Development of Cataracts | Glaucoma | Retinal vein occlusion |
| Ischemia caused by anterior optic nerve | Ocular ischemic syndrome | Retinal arteriolar emboli |
| Diabetic papillopathy | Retinal artery occlusion | |
| Ocular movement disorders | Corneal diseases |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55090546
Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina. 2019; 55(9):546. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55090546
Chicago/Turabian StyleKhan, Radia Marium Modhumi, Zoey Jia Yu Chua, Jia Chi Tan, Yingying Yang, Zehuan Liao, and Yan Zhao. 2019. "From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research" Medicina 55, no. 9: 546. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina55090546





