1. Introduction
Percutaneous ablation of primary and secondary liver lesions constitutes a well-established therapeutic technique for the management of hepatocellular carcinoma or hepatic metastases of various neoplasmatic origin [
1,
2,
3]. Almost all diagnostic methods have been used either solely or in combination (with or without fusion imaging) for ablation guidance [
4]. In the vast majority of ablation sessions, a conventional manual free-hand approach is performed; more recently, however, a stereotactic computer-assisted navigation has been applied, aiming to improve the precision of the needle placement [
5,
6,
7,
8,
9,
10]. The CT-navigation system allows for real-time treatment planning during the procedure and helps to avoid critical structures without angular limitations for an optimal trajectory [
11]. General anesthesia or deep sedation seems to be a prerequisite for the application of this stereotactic, computer-assisted navigation [
5,
6,
7,
8,
9,
10,
11]. Unfortunately, although anesthesiologists are best trained, they are not available to attend all ablation procedures [
12].
The anatomical location of a hepatic tumor significantly affects local recurrence post percutaneous ablation [
13]. Challenging locations for tumor ablation in the liver include, among others, the hepatic dome, areas close to the liver hilum or to the heart, and subcapsular locations [
14]. In the hepatic dome, apart from the approach of a needle which is angulated in the vast majority of the cases (increasing the difficulty level), the presence of the diaphragm results in a shift towards a conservative ablation strategy. Although this serves as an attempt to avoid complications from either direct penetration or more commonly thermal trauma, it can result in under-treatment [
15].
The aim of the present study was to report the safety and efficacy of percutaneous navigation under local anesthesia for computed tomography-guided microwave ablation of malignant liver lesions located in the hepatic dome.
2. Materials and Methods
2.1. Patient Selection and Evaluation
The present study is a prospective observational study evaluating patients with primary and secondary malignant liver lesions located in the hepatic dome; all lesions were treated with percutaneous computed tomography-guided microwave ablation using a computer-assisted navigation system under local anesthesia. The primary objective was technical success. Secondary objectives included an evaluation of complications. All included lesions were evaluable for the 3-month follow-up. Inclusion criteria included patients ≥18 years old with primary and secondary malignant liver lesions located in the hepatic dome, coagulation parameters within normal limits, and a life expectancy of >3 months. Exclusion criteria included non-compliance of patients, uncontrollable INR, systematic or local infection, expected survival less than 3 months, ECOG score less than 3, and presence of a medical or psychiatric illness that would preclude informed consent or follow-up. Each patient underwent laboratory tests at least 24 h prior to the percutaneous ablation session. The patients were fully informed about the procedure, the possible complications, and the surgical alternatives available; informed written consent for both the technique and the study was obtained in all cases. Indication for microwave ablation was determined at a multidisciplinary tumor board. Patient characteristics, ablation and navigation technique, efficacy, and complications were evaluated.
2.2. Percutaneous Computed Tomography-Guided Microwave Ablation Using a Stereotactic, Computer-Assisted Navigation System
According to directions provided by the Infection Division of Pathology Department, prophylactic antibiotic was intravenously administered 45–60 min before MWA session and repeated twice over 24 h. A single operator with 12 years of experience performed all ablation sessions; the operator’s experience using the percutaneous computer-assisted navigation system was only 3 months. Microwave ablation was always performed in an inpatient setting under local anesthesia (10 cc of 2% Lidocaine Hydrochloric on skin and subcutaneous tissues) and intravenous analgesia (1 gr paracetamol and 100 mg of tramadol diluted in 100 mL of normal saline were administered during the procedure). Under local sterility, microwave ablation was performed with the percutaneous approach in all lesions.
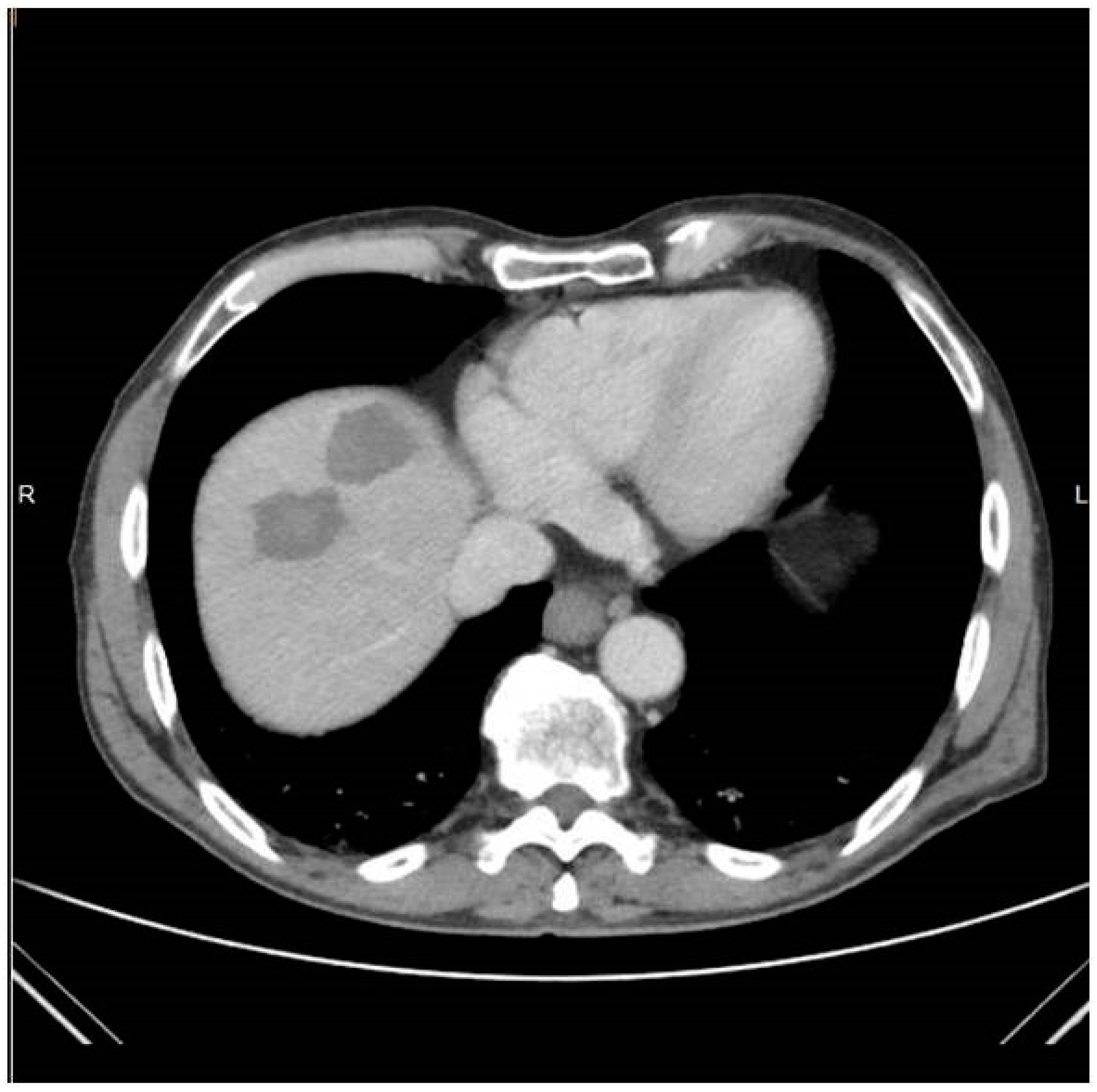
Trajectory planning and insertion of the microwave antenna were conducted using a commercially available navigation system for interventional radiology (IMACTIS SAS, Saint Martin d’Hères, France), (
Figure 1). Educating the patient for correct breathing is of outmost importance; in the present study, all scans (both set-up and control ones) as well as needle movements were performed in the end-expiration apnea. In all lesions included in the present study, ablation was performed using a single microwave antenna. Once in the correct location, the ablation session was set up and performed according to the coagulation charts provided by the manufacturer in consideration of the tumor size and location and the desired safety margin. In all sessions, track ablation was performed during antenna removal from the liver in order to reduce potential risk of bleeding and peritoneal tumor seeding. CT scan in the arterial and portal venous phases validated the ablation zone and evaluated any potential immediate complications at the end of the MWA treatment (
Figure 2). All patients were hospitalized overnight.
2.3. Outcome Measures
Technical success was defined as successful completion of the planned microwave ablation of each target lesion. Treatment outcome was reported based on standard reporting criteria [
16]. Patients had a follow-up imaging with either contrast-enhanced CT or MRI at 1 and 3 months after the ablation combined with a consultation. Patients with a recurrent or residual disease were consulted for their options. Patient demographics (age, sex) as well as tumor characteristics, microwave technique, pattern of recurrence, and survival rate were evaluated. Technical success was defined as complete tumor necrosis after a single microwave ablation session with no evidence of tumor remnant or recurrence on subsequent cross-sectional imaging [
17]. Progression-free survival was defined as the time interval post MWA without evidence of local recurrence. Recurrence-free survival rate was defined as the time elapsed between the intervention and any recurrence (local, regional, or distant). The definition of complications was assigned according to the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification system [
18].
2.4. Statistical Analysis
Patient characteristics and results were presented by means of descriptive statistics. Total number, percentage, mean and standard deviation, or median and range were used to present continuous data. Analyses were performed using acommercially available software (SPSS version 25).
3. Results
Demographic and clinical data of patients and lesions included in the present study are presented in
Table 1. The sample consisted by 10 participants (16 lesions). The mean age of the included patients was 60.60 years (SD = 9.25 years). Neoplasmatic substrate included: hepatocellular carcinoma [2/10 (20%)], colorectal carcinoma [4/10 (40%)], gastrointestinal stromal tumor [2/10 (20%)], and myeloid carcinoma of the thyroid gland [2/10 (20%)]. The mean size of the lesions was 20.37 ± 7.29 mm, and maximum tumor size ranged from 8 to 30 mm Mean follow-up time was 3.4 months (SD = 1.41) months. Most of the participants were males, with the percentage being 80%. In the 2 HCC patients’ percutaneous ablation was decided in the multidisciplinary tumor board meeting as a first-line therapy due to co-morbidities, lesion location, and size (< 3cm in diameter). As far as the four patients with colorectal cancel liver metastases are concerned, ¾ patients involved metachronous lesions post surgery of the original intestinal tumor and systemic chemotherapy and ¼ patients suffered from synchronous hepatic lesions. In this last patient, percutaneous ablation was performed post systemic chemotherapy, whilst surgical operation of the intestinal tumor was performed the morning post ablation. In the remaining 4/10 patients, percutaneous ablation was performed in metachronous metastatic disease resistant to systemic therapies and post surgical operation of the original tumor. Technical success was 100% (i.e., antenna placement at the target lesion was successful in all patients). There was no need for hydrodissection or any other ancillary methods.
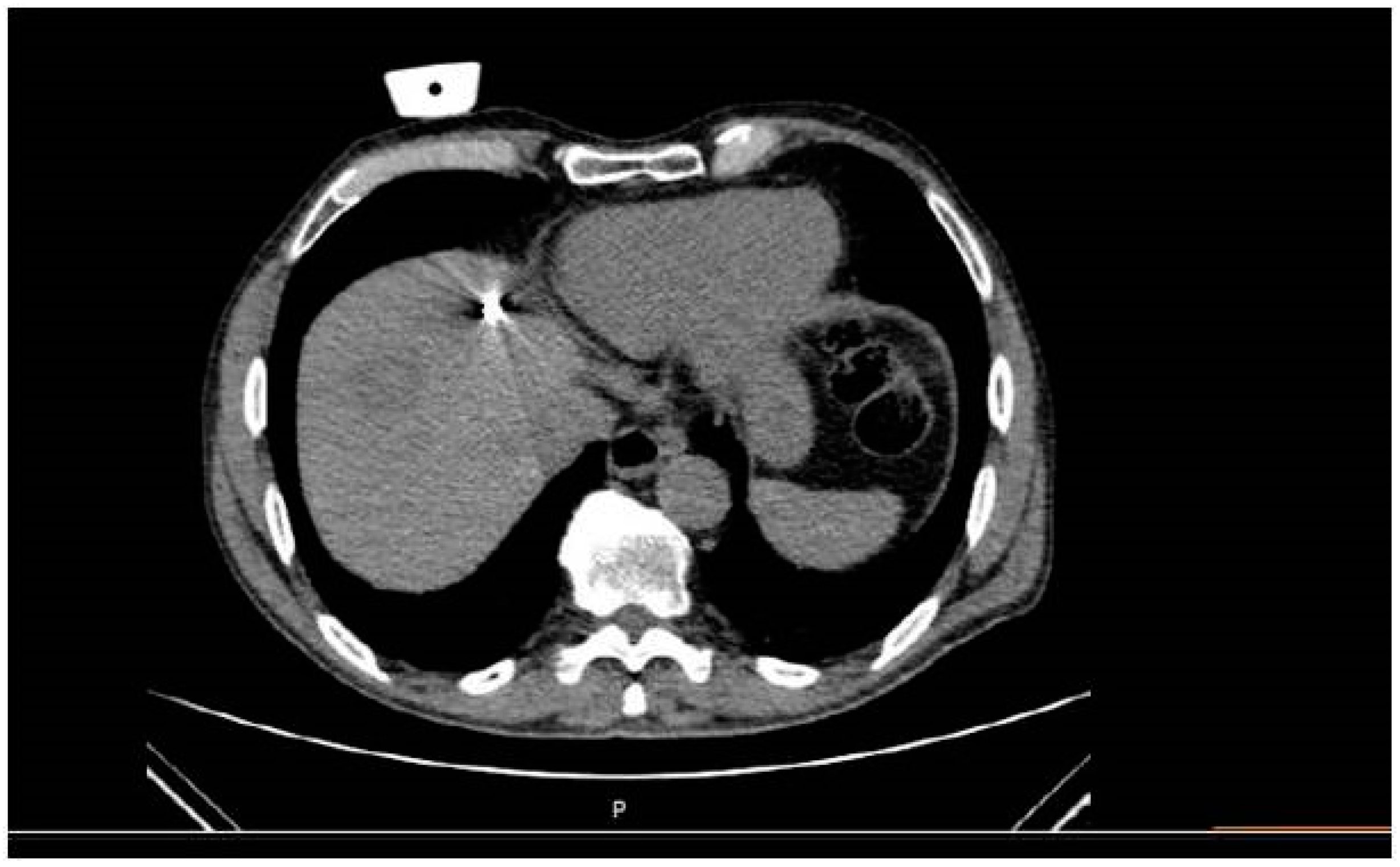
The mean total duration of the procedure from entrance to exit of the patient was 49.45 (SD = 7.53 min). Specifically, a median of 7 min was necessary for planning time and 12 min for insertion time. Whenever deemed necessary (4/16 sessions), the microwave antenna was re-positioned and a second ablation session was performed, so as to ensure that the final ablation completely encompassed both the target tumor and an annular safety zone around it that was a minimum of 5 mm thick. Navigation was used for all four repositioning. A median of 11 scans was performed including planning and control scans as well as a scan during ablation (
Figure 3) and immediate imaging follow-up with 3 scans (prior to and post contrast medium injection in the arterial and portal venous phases).
On a per lesion basis, tumor remnant was noticed at one month follow-up in a single metastatic lesion (from myeloid carcinoma of the thyroid gland) (1/16, primary technical success 93.75%). This lesion was re-treated with an ablation session and no tumor remnant was depicted in the subsequent imaging follow-up (secondary technical success 100%). On a per patient basis, no disease progression was depicted in or outside the liver. Grade I self-limited complications (according to the CIRSE classification system) included small pleural effusion (n = 1) and active extravasation post antenna removal (n = 1) requiring nothing but observation. In all cases, both imaging and clinical control was performed in the first 24 h confirming the lack of a need for any further actions.
4. Discussion
The main problematic issue in ablation of liver tumors is incomplete ablation. Pre-requisites contributing to the prevention of incomplete ablation include the location and diameter of the tumor, proximity to vessels, accurate positioning of the ablation antenna(s) in the tumor, sufficient energy deposition, and lastly evaluation of the ablation zone. The present study adds to the growing number of case series showing that percutaneous microwave ablation under stereotactic navigation is feasible, safe, and efficacious for the treatment of malignant liver lesions [
5,
6,
7,
8,
9,
10,
11]. Perodin et al. retrospectively evaluated 23 patients (40 liver lesions) undergoing percutaneous stereotactic imaging-guided microwave ablation, reporting an incomplete ablation rate of only 2.5% [
5]. Schaible et al. retrospectively evaluated 221 patients (423 liver lesions), comparing stereotactic and conventional manual guidance. The authors concluded that percutaneous microwave ablation under stereotactic guidance exhibited significantly greater primary efficacy than conventional manual guidance [
6]. Tingueli et al. investigated factors influencing the targeting accuracy and treatment efficacy of percutaneous stereotactic image-guided microwave ablation for malignant liver neoplasms, concluding that even for lesions in challenging locations, navigation allows precise and effective treatment, expanding treatment eligibility for patients with otherwise difficult to target tumors [
7]. In the present study, all treated lesions were located in a challenging location (hepatic dome) and the primary technical efficacy was 93.75%, with successful ablation in 15/16 target lesions. Correct antenna placement in the lesion target (which was feasible in all cases despite the difficulty level raised by the challenging location) along with the reported technical efficacy rate provide preliminary evidence that the present concept is technically possible, functional, and producible and can act as a working model.
There are different devices for percutaneous needle guidance. These needle placement systems can be divided into two groups: (a) active guidance needle placement (mounted on patient, table, gantry, floor), and (b) passive guidance needle placement (additional feedback of needle position). The system used in the present study can be classified as a patient-mounted device that is positioned on the external skin surface of the patient during the procedure. In the literature, these devices have already been described and discussed; however, in all reported cases, navigation guidance and ablation were performed under deep sedation or general anesthesia (including jet ventilation). Similar to other studies, in the present case series, the treatment of malignant liver lesions with stereotactic navigation of microwave ablation was successful and well tolerated. One major difference of the present study is that all patients were treated under local anesthesia combined with intravenous analgesia, resulting, however, in no significant differences concerning the efficacy and safety rates. To our knowledge, the present study is the first one evaluating the performance of a patient-mounted device for navigation of tumor ablation under local anesthesia.
Limitations of the present study include the small number of participants and the lack of comparison to a group of patients undergoing alternative (i.e., guidance and ablation performed under deep sedation or general anesthesia) approaches. Furthermore, from an oncologic point of view, including in the same patient pool various oncologic substrates limits the validity of the survival rates; however, the present study aimed to primarily focus upon the feasibility and technical efficacy of performing percutaneous navigation under local anesthesia for computed tomography-guided microwave ablation of malignant liver lesions located in the hepatic dome.
5. Conclusions
In conclusion, the findings of the present study indicate that percutaneous navigation under local anesthesia is a safe and efficacious approach for computed tomography-guided microwave ablation of malignant liver lesions located in the hepatic dome. Large randomized controlled studies are warranted to observe treatment effectiveness and compare the results with those of other options.
Author Contributions
Conceptualization, D.K.F.; methodology, D.K.F., G.V. and A.M.; validation, D.K.F., N.K. and A.K.; formal analysis, D.K.F., G.V. and A.M.; investigation, T.V. and A.T.; resources, D.K.F.; data curation, D.K.F., N.K. and A.K.; writing—original draft preparation, D.K.F., T.V. and A.T.; writing—review and editing D.K.F., N.K. and A.K.; visualization, D.K.F.; supervision, N.K., A.K. and D.K.F.; project administration, A.M., G.V. and D.K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of University General Hospital “Attikon” (Aktin Ebd 467/02-07-2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (Version 1.2020). Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 21 January 2020).
- Crocetti, L.; De Baére, T.; Pereira, P.L.; Tarantino, F.P. CIRSE Standards of Practice on Thermal Ablation of Liver Tumours. Cardiovasc. Interv. Radiol. 2020, 43, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.; Velonakis, G.; Kelekis, A.; Sofocleous, C. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Perrodin, S.; Lachenmayer, A.; Maurer, M.; Kim-Fuchs, C.; Candinas, D.; Banz, V. Percutaneous stereotactic image-guided microwave ablation for malignant liver lesions. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaible, J.; Lürken, L.; Wiggermann, P.; Verloh, N.; Einspieler, I.; Zeman, F.; Schreyer, A.G.; Bale, R.; Stroszczynski, C.; Beyer, L. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: Comparison of stereotactic and conventional manual guidance. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, P.; Frehner, L.; Lachenmayer, A.; Banz, V.; Weber, S.; Candinas, D.; Maurer, M.H. Stereotactic Image-Guided Microwave Ablation for Malignant Liver Tumors—A Multivariable Accuracy and Efficacy Analysis. Front. Oncol. 2020, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Lachenmayer, A.; Tinguely, P.; Maurer, M.H.; Frehner, L.; Knöpfli, M.; Peterhans, M.; Weber, S.; Dufour, J.F.; Candinas, D.; Banz, V. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int. 2019, 39, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Johnston, E.; Laimer, G.; Putzer, D.; Eberle, G.; Scharll, Y.; Ianetti-Hackl, C.; Bale, R. Stereotactic Radiofrequency Ablation of Breast Cancer Liver Metastases: Short- and Long-Term Results with Predicting Factors for Survival. Cardiovasc. Interv. Radiol. 2021, 4, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Johnston, E.W.; Putzer, D.; Eberle, G.; Laimer, G.; Bale, R. Stereotactic radiofrequency ablation of subcardiac hepatocellular carcinoma: A case-control study. Int. J. Hyperth. 2019, 36, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Rouchy, R.; Moreau-Gaudry, A.; Chipon, E.; Aubry, S.; Pazart, L.; Lapuyade, B.; Durand, M.; Hajjam, M.; Pottier, S.; Renard, B.; et al. Evaluation of the clinical benefit of an electromagnetic navigation system for CT-guided interventional radiology procedures in the thoraco-abdominal region compared with conventional CT guidance (CTNAV II): Study protocol for a randomised controlled trial. Trials 2017, 18, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelis, F.H.; Monard, E.; Moulin, M.A.; Vignaud, E.; Laveissiere, F.; Ben Ammar, M.; Nouri-Neuville, M.; Barral, M.; Lombart, B. Sedation and analgesia in interventional radiology: Where do we stand, where are we heading and why does it matter? Diagn. Interv. Imaging 2019, 100, 753–762. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local recurrence after hepatic radiofrequency coagulation: Multivariate meta-analysis and review of contributing factors. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Spiliopoulos, S.; Konstantos, C.; Reppas, L.; Kelekis, A.; Brountzos, E.; Kelekis, N. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: Safety and efficacy of high-power microwave platforms. Int. J. Hyperth. 2017, 34, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Howenstein, M.J.; Sato, K.T. Complications of Radiofrequency Ablation of Hepatic, Pulmonary, and Renal Neoplasms. Semin. Interv. Radiol. 2010, 27, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baère, T.; Dodd, G.D., 3rd; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef] [PubMed]
- An, T.J.; Arellano, R.S. Comparison of Safety and Efficacy of Percutaneous Microwave Ablation of Central Versus Peripheral Renal Cell Carcinoma. Cardiovasc. Interv. Radiol. 2020, 44, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).