Revisiting a Distinct Entity in Pulmonary Vascular Disease: Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
Abstract
:1. Introduction
2. Epidemiology
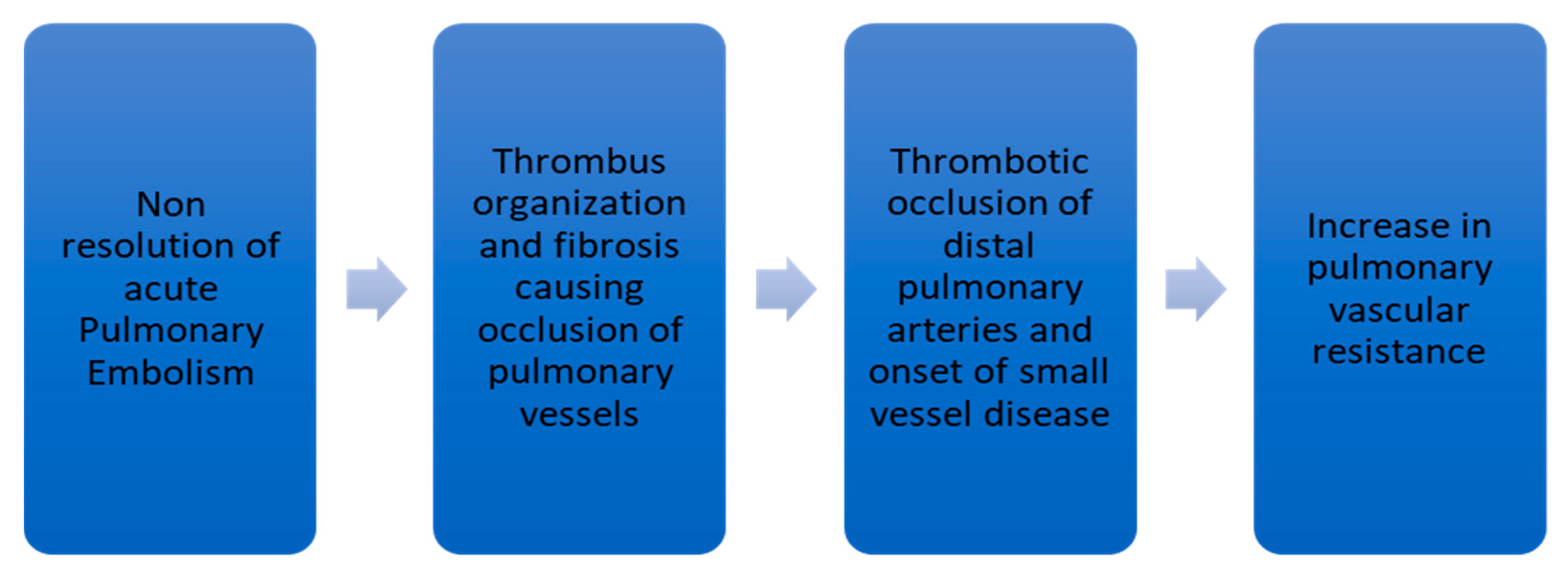
3. Pathophysiology of CTEPH
4. Predisposing Factors for CTEPH
5. Clinical Features
6. Diagnostic Evaluation in CTEPH
6.1. Ventilation-Perfusion Lung Scan, Computed Tomography Pulmonary Angiography, and Magnetic Resonance Angiography in CTEPH
6.2. Role of Right Heart Catheterization and Pulmonary Angiography
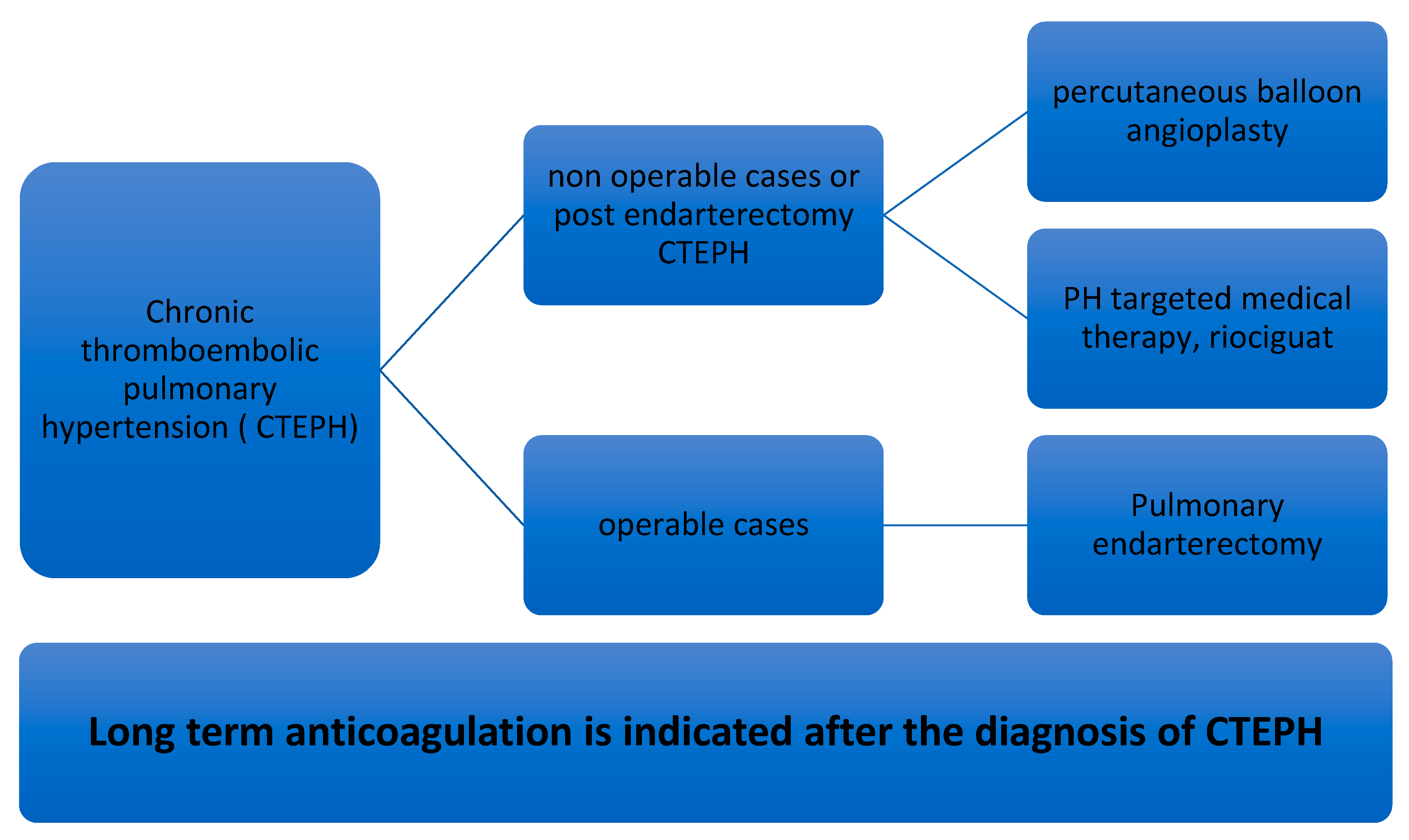
7. Management of CTEPH
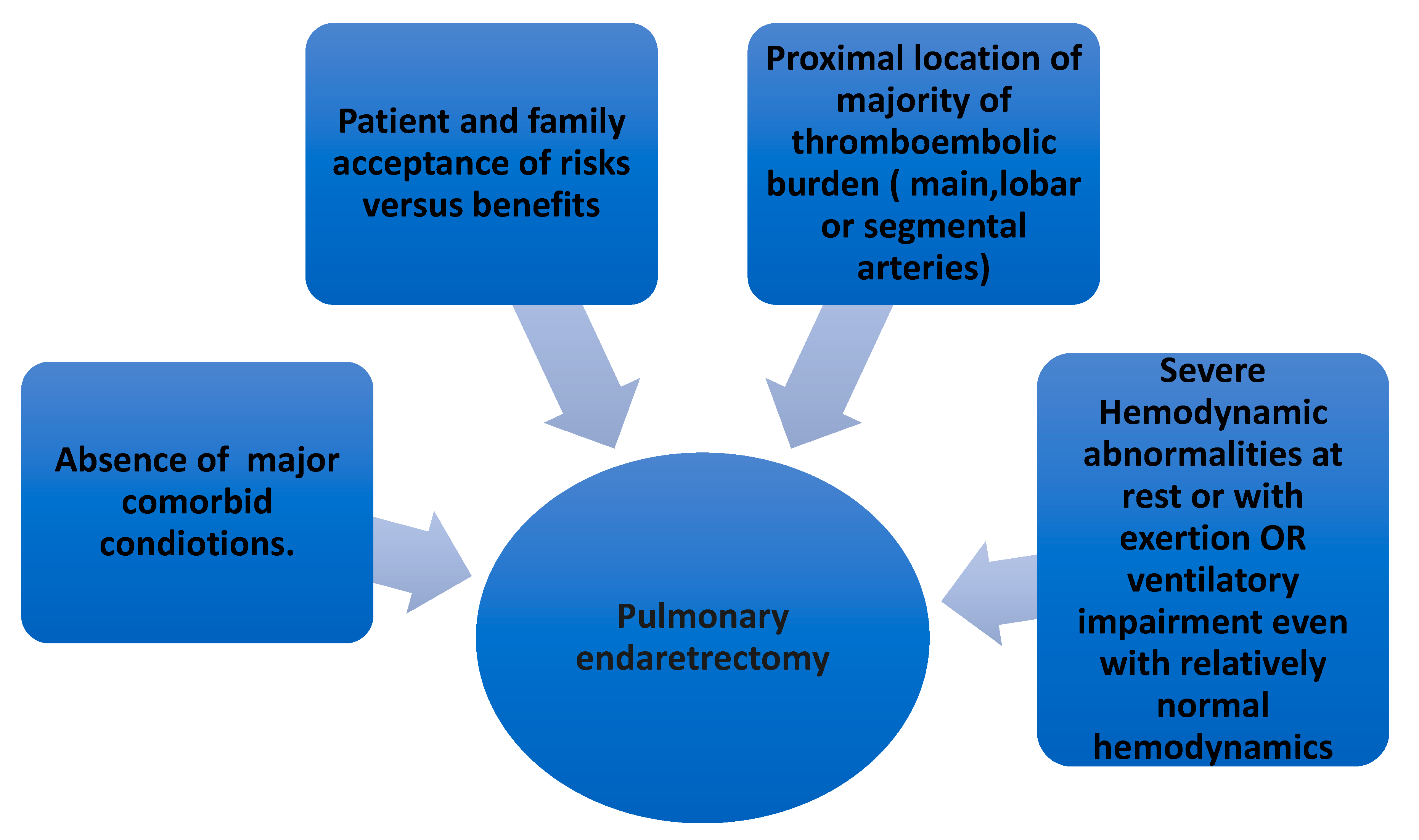
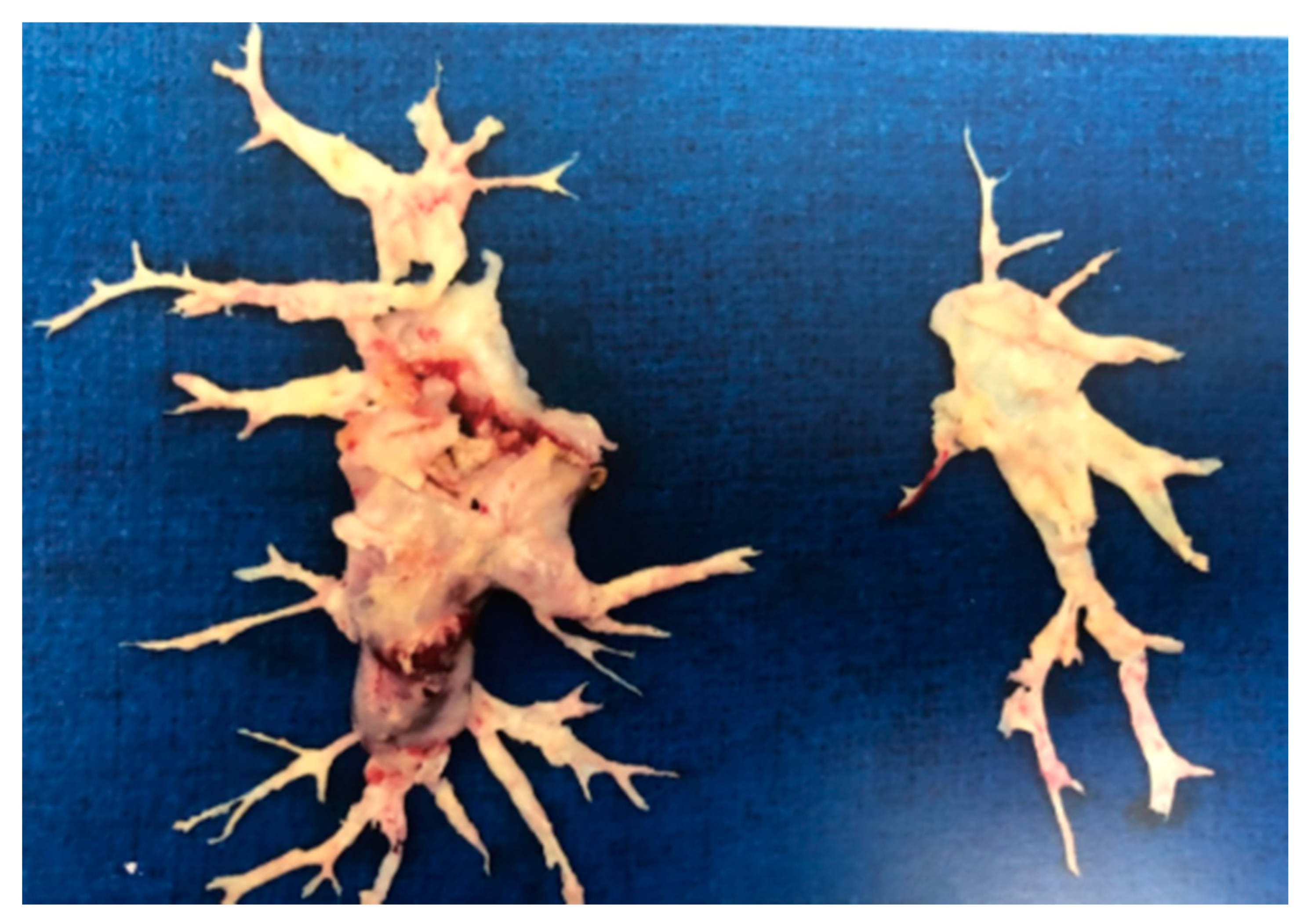
7.1. Pulmonary Thromboendarterectomy (PEA) in CTEPH
7.2. Percutaneous Pulmonary Balloon Angioplasty (BPA) in CTEPH
7.3. PH-Targeted Medical Therapy in CTEPH
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Fedullo, P.F. Overview of the Treatment of Chronic Thromboembolic Pulmonary Hypertension. Available online: https://www.uptodate.com/contents/overview-of-the-treatment-of-chronic-thromboembolic-pulmonary-hypertension (accessed on 21 February 2021).
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Noordegraaf, A.V.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef]
- Miniati, M.; Monti, S.; Bottai, M.; Scoscia, E.; Bauleo, C.; Tonelli, L.; Dainelli, A.; Giuntini, C. Survival and Restoration of Pulmonary Perfusion in a Long-Term Follow-Up of Patients after Acute Pulmonary Embolism. Medicine 2006, 85, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; van Kralingen, K.W.; van Dijk, A.P. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010, 95, 970. [Google Scholar] [CrossRef]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; Albanese, P.; Biasiolo, A.; Pegoraro, C.; Iliceto, S.; et al. Incidence of Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism. N. Engl. J. Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghaus, T.M.; Barac, M.; Von Scheidt, W.; Schwaiblmair, M. Echocardiographic evaluation for pulmonary hypertension after recurrent pulmonary embolism. Thromb. Res. 2011, 128, e144–e147. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, A.; Satoh, T.; Fukuda, T. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H. Group 4 pulmonary hypertension: Chronic thromboembolic pulmonary hypertension: Epidemiology, pathophysiology, and treatment. Cardiol. Clin. 2016, 34, 435–441. [Google Scholar] [CrossRef]
- Wagenvoort, C.A. Pathology of pulmonary thromboembolism. Chest 1995, 107, 10S–17S. [Google Scholar] [CrossRef] [Green Version]
- Banks, D.A.; Pretorius, G.V.; Kerr, K.M. Pulmonary endarterectomy: Part I. Pathophysiology, clinical manifestations, and diagnostic evaluation of chronic thromboembolic pulmonary hypertension. Semin. Cardiothorac. Vasc. Anesth 2014, 18, 319–330. [Google Scholar] [CrossRef]
- Lang, I.M.; Dorfmüller, P.; Noordegraaf, A.V. The Pathobiology of Chronic Thromboembolic Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2016, 13, S215–S221. [Google Scholar] [CrossRef]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schafers, H.-J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2008, 33, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonderman, D.; Jakowitsch, J.; Redwan, B.; Bergmeister, H.; Renner, M.-K.; Panzenböck, H.; Adlbrecht, C.; Georgopoulos, A.; Klepetko, W.; Kneussl, M.; et al. Role for Staphylococci in Misguided Thrombus Resolution of Chronic Thromboembolic Pulmonary Hypertension. Arter. Thromb. Vasc. Biol. 2008, 28, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Zabini, D.; Heinemann, A.; Foris, V.; Nagaraj, C.; Nierlich, P.; Bálint, Z.; Kwapiszewska, G.; Lang, I.M.; Klepetko, W.; Olschewski, H.; et al. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur. Respir. J. 2014, 44, 951–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarck, R.; Nawrot, T.; Meyns, B. C-reactive protein: A new predictor of adverse outcome in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 53, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Boyer-Neumann, C.; Parent, F.; Eschwege, V.; Jaillet, H.; Meyer, D.; Simonneau, G. Thrombotic risk factors in pulmonary hypertension. Eur. Respir. J. 2000, 15, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Turecek, P.L.; Jakowitsch, J.; Weltermann, A.; Adlbrecht, C.; Schneider, B.; Kneussl, M.; Rubin, L.J.; Kyrle, P.A.; Klepetko, W.; et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2003, 90, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Su, P.; Yan, J.; Zhang, X.; An, X.; Gao, J.; Xin, R.; Liu, Y. Comparison of gene expression profiles and related pathways in chronic thromboembolic pulmonary hypertension. Int. J. Mol. Med. 2013, 33, 277–300. [Google Scholar] [CrossRef] [Green Version]
- Lindner, J.; Maruna, P.; Kunstyr, J.; Jansa, P.; Gürlich, R.; Kubzova, K.; Zakharchenko, M.; Linhart, A. Hemodynamic Instability after Pulmonary Endarterectomy for Chronic Thromboembolic Pulmonary Hypertension Correlates with Cytokine Network Hyperstimulation. Eur. Surg. Res. 2009, 43, 39–46. [Google Scholar] [CrossRef]
- Le Gal, G.; Delahousse, B.; Lacut, K.; Malaviolle, V.; Regina, S.; Blouch, M.T.; Couturaud, F.; Mottier, D.; Oger, E.; Gruel, Y.; et al. Fibrinogen Aα-Thr312Ala and factor XIII-A Val34Leu polymorphisms in idiopathic venous thromboembolism. Thromb. Res. 2007, 121, 333–338. [Google Scholar] [CrossRef]
- Yaoita, N.; Shirakawa, R.; Fukumoto, Y.; Sugimura, K.; Miyata, S.; Miura, Y.; Nochioka, K.; Miura, M.; Tatebe, S.; Aoki, T.; et al. Platelets Are Highly Activated in Patients of Chronic Thromboembolic Pulmonary Hypertension. Arter. Thromb. Vasc. Biol. 2014, 34, 2486–2494. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Delcroix, M.; Jais, X. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, R.; Kiely, D.G.; Gibbs, J.S.R.; Corris, P.A.; Peacock, A.J.; Jenkins, D.P.; Goldsmith, K.; Coghlan, J.G.; Pepke-Zaba, J. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2008, 33, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedullo, P.F. Clinical Manifestations and Diagnosis of Chronic Thromboembolic Pulmonary Hypertension; Finlay, G., Ed.; Uptodate: Waltham, MA, USA, 22 January 2016. [Google Scholar]
- Arunthari, V.; Burger, C.D. Utility of D-Dimer in the Diagnosis of Patients with Chronic Thromboembolic Pulmonary Hypertension. Open Respir. Med. J. 2009, 3, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, D.X.; Coates-Bradshaw, L.D.; Willis, J.; Harkness, A.; Ring, L.; Grapsa, J.; Coghlan, G.; Kaye, N.; Oxborough, D.; Robinson, S.; et al. Echocardiographic assessment of pulmonary hypertension: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2018, 5, G11–G24. [Google Scholar] [CrossRef] [Green Version]
- Raisinghani, A.; Ben-Yehuda, O. Echocardiography in Chronic Thromboembolic Pulmonary Hypertension. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 230–235. [Google Scholar] [CrossRef]
- Tunariu, N.; Gibbs, S.J.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; Al-Nahhas, A. Ventilation-Perfusion Scintigraphy Is More Sensitive than Multidetector CTPA in Detecting Chronic Thromboembolic Pulmonary Disease as a Treatable Cause of Pulmonary Hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.L.; Fedullo, P.F.; Davis, G.B.; Vasquez, T.E.; Moser, K.M. Perfusion Scan Findings Understate the Severity of Angiographic and Hemodynamic Compromise in Chronic Thromboembolic Pulmonary Hypertension. Chest 1988, 93, 1180–1185. [Google Scholar] [CrossRef]
- Rajaram, S.; Swift, A.J.; Telfer, A.; Hurdman, J.; Marshall, H.; Lorenz, E.; Capener, D.; Davies, C.; Hill, C.; Elliot, C.; et al. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: Results from the ASPIRE Registry. Thorax 2013, 68, 677–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitton, M.B.; Düber, C.; Mayer, E.; Thelen, M. Hemodynamic Effects of Nonionic Contrast Bolus Injection and Oxygen Inhalation during Pulmonary Angiography in Patients with Chronic Major-Vessel Thromboembolic Pulmonary Hypertension. Circulation 1996, 94, 2485–2491. [Google Scholar] [CrossRef] [Green Version]
- Piazza, G.; Goldhaber, S.Z. Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2011, 364, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Fedullo, P.; Kerr, K.M.; Kim, N.H.; Auger, W.R. Chronic Thromboembolic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- A Thistlethwaite, P.; Kaneko, K.; Madani, M.M.; Jamieson, S.W. Technique and outcomes of pulmonary endarterectomy surgery. Ann. Thorac. Cardiovasc. Surg. 2008, 14, 274–282. [Google Scholar]
- Taboada, D.; Pepke-Zaba, J.; Jenkins, D.P.; Berman, M.; Treacy, C.M.; Cannon, J.E.; Toshner, M.; Dunning, J.J.; Ng, C.; Tsui, S.S.; et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur. Respir. J. 2014, 44, 1635–1645. [Google Scholar] [CrossRef]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J. Long-Term Outcome of Patients with Chronic Thromboembolic Pulmonary Hypertension: Results from an International Prospective Registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.E.; Su, L.; Kiely, D.G. Dynamic Risk Stratification of Patient Long-Term Outcome after Pulmonary Endarterectomy: Results from the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, T.; Ogawa, A.; Miyaji, K. Novel Angiographic Classification of Each Vascular Lesion in Chronic Thromboembolic Pulmonary Hypertension Based on Selective Angiogram and Results of Balloon Pulmonary Angioplasty. Circ. Cardiovasc. Interv. 2016, 9, e003318. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonneau, G.; D’Armini, A.M.; Ghofrani, H.-A.; Grimminger, F.; Jansa, P.; Kim, N.H.; Mayer, E.; Pulido, T.; Wang, C.; Colorado, P.; et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: Data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir. Med. 2016, 4, 372–380. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Simonneau, G.; D’Armini, A.M.; Fedullo, P.; Howard, L.S.; Jaïs, X.; Jenkins, D.P.; Jing, Z.C.; Madani, M.M.; Martin, N.; et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): Results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir. Med. 2017, 5, 785–794. [Google Scholar] [CrossRef]
- Jaïs, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Mayer, E.; Pepke-Zaba, J.; et al. Bosentan for Treatment of Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.W.; Kerr, K.M.; Fedullo, P.F.; Kim, N.H.; Test, V.J.; Ben-Yehuda, O.; Auger, W.R. Pulmonary Hypertensive Medical Therapy in Chronic Thromboembolic Pulmonary Hypertension before Pulmonary Thromboendarterectomy. Circulation 2009, 120, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group 1 | Pulmonary Arterial Hypertension (PAH) |
| Group 2 | Due to Left Heart Disease |
| Group 3 | Due to Chronic Lung Disease/Hypoxemia |
| Group 4 | Due to Pulmonary Arterial Obstructions |
| Group 5 | Multifactorial |
| Investigators | Year of Publication | Type of Study | Main Results |
|---|---|---|---|
| Ende-Verhaar YM et al. [3] | February 2017 | Metanalysis of patients with PE followed up for CTEPH. Sample size n: 4047 patients from 16 studies | -CTEPH incidence was 3.2% (95% CI 2–4.4) of 999 patients who survived after PE ≥ 2 years (4 studies) -Pooled CTEPH incidence 0.56% (95% CI 0.1–1.0) (3 studies) -In survivors without major comorbidities, incidence was 2.8% in 1775 patients (9 studies) |
| Miniati M et al. [4] | September 2006 | Prospective study of 320 patients with proven PE follow-up for a median duration of 2.1 years | 4 out of 320 patients with proven PE (1%) developed CTEPH |
| Klok FA et al. [5] | January 2010 | Cohort screening study in 866 patients with acute PE studied between January 2001 and July 2007 | CTEPH incidence was 0.57% (95% CI, 0.02–1.2%) in all-cause PE and 1.5% (95% CI, 0.08–3.1%) in provoked PE |
| Pengo V. [6] | May 2004 | Prospective follow-up of 223 patients with acute PE for median duration of 94.3 months. Follow-up ventilation-perfusion scan and pulmonary angiography done in patients suspected to have CTEPH | CTEPH incidence was 1% (95% CI, 0.0–2.4) at 6 months, 3.1% (95% CI, 0.7–5.5) at 1 year, and 3.8% (95 CI, 1.1–6.5) at 2 years |
| Berghaus TM [7] | December 2011 | Cohort screening study of 43 survivors of recurrent PE | CTEPH in 5 patients (11.6%) patients with recurrent PE |
| Possible Risk Factors for CTEPH |
|---|
| (1) Large pulmonary emboli |
| (2) Recurrent pulmonary emboli |
| (3) Insufficient anticoagulation |
| (4) Underlying cancer |
| (5) Chronic inflammatory states |
| (6) Infections (example: staphylococcal infection) |
| (7) Biological and genetic risk factors |
| (8) Blood groups |
| (9) Abnormal fibrinogen levels and fibrinolysis |
| (10) Platelet dysfunction |
| (11) Platelet endothelial adhesion molecule deficiency (PECAM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Levine, D.J. Revisiting a Distinct Entity in Pulmonary Vascular Disease: Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Medicina 2021, 57, 355. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57040355
Sharma M, Levine DJ. Revisiting a Distinct Entity in Pulmonary Vascular Disease: Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Medicina. 2021; 57(4):355. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57040355
Chicago/Turabian StyleSharma, Munish, and Deborah Jo Levine. 2021. "Revisiting a Distinct Entity in Pulmonary Vascular Disease: Chronic Thromboembolic Pulmonary Hypertension (CTEPH)" Medicina 57, no. 4: 355. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57040355





