Prognostic Value of Cortisol Index of Endobiogeny in Acute Myocardial Infarction Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Samples
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Onset of Pain to Admission Time
3.3. Serum Cortisol on Admission and Cortisol Index Variation during and after AMI
3.4. Serum Cortisol and Cortisol Index on Admission Correlation with Some Inflammatory Markers
3.5. Correlation between Troponin I with Serum Cortisol, Cortisol Index and ∆Cortisol Index
3.6. Serum Cortisol, Cortisol Index and Troponin I Results and Mortality
3.7. RR of Mortality in Serum Cortisol with Troponin I Group and Cortisol Index with Troponin I Group
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | acute myocardial infarction |
| AUC—ROC | area under the Receiver Operating Characteristics |
| CBCD | a complete blood count with differential |
| CI | confidence interval |
| CKD | chronic kidney disease |
| COPD | chronic obstructive pulmonary disease |
| CVA | cerebrovascular accident |
| M | Mean |
| MI | myocardial infarction |
| NYHA | the New York Heart Association |
| NSTEMI | non ST—elevation myocardial infarction |
| ROC | Receiver Operating Characteristics |
| RR | Relative Risk |
| STEMI | ST—elevation myocardial infarction |
| SD | Standard Deviation |
| 11βHSD | 11β-hydroxysteroid dehydrogenase |
References
- Stillman, A.E.; Oudkerk, M.; Bluemke, D.; Bremerich, J.; Esteves, F.P.; Garcia, E.V.; Gutberlet, M.; Hundley, W.G.; Jerosch-Herold, M.; Kuijpers, D.; et al. Assessment of acute myocardial infarction: Current status and recommendations from the North American society for cardiovascular imaging and the European society of cardiac radiology. Int. J. Cardiovasc. Imaging 2010, 27, 7–24. [Google Scholar] [CrossRef] [Green Version]
- McManus, D.D.; Gore, J.; Yarzebski, J.; Spencer, F.; Lessard, D.; Goldberg, R.J. Recent Trends in the Incidence, Treatment, and Outcomes of Patients with STEMI and NSTEMI. Am. J. Med. 2011, 124, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Rosamond, W.D.; Chambless, L.E.; Heiss, G.; Mosley, T.H.; Coresh, J.; A Whitsel, E.; E Wagenknecht, L.; Ni, H.; Folsom, A.R. Twenty-Two–Year Trends in Incidence of Myocardial Infarction, Coronary Heart Disease Mortality, and Case Fatality in 4 US Communities, 1987–2008. Circulation 2012, 125, 1848–1857. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Daubert, M.A.; Jeremias, A. The utility of troponin measurement to detect myocardial infarction: Review of the current find-ings. Vasc. Health Risk Manag. 2010, 6, 691–699. [Google Scholar]
- Hallén, J. Troponin for the Estimation of Infarct Size: What Have We Learned. Cardiology 2012, 121, 204–212. [Google Scholar] [CrossRef]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Hosbond, S.; Jangaard, L.S.; Diederichsen, A.C.; Thygesen, K.; Mickley, H. Prognostic Impact of Myocardial Injury Related to Various Cardiac and Noncardiac Conditions. Am. J. Med. 2016, 129, 506–514.e1. [Google Scholar] [CrossRef] [Green Version]
- De la Hoz, R.A.; Swieszkowski, S.P.; Cintora, F.M.; Aladio, J.M.; Papini, C.M.; Matsudo, M.; Scazziota, A.S. Neuroendocrine System Regulatory Mechanisms: Acute Coronary Syndrome and Stress Hyperglycaemia. Eur. Cardiol. 2018, 13, 29–34. [Google Scholar] [CrossRef]
- Hedayat, K.; Lapraz, J.C. The Theory of Endobiogeny: Global Systems Thinking and Biological Modeling for Clinical Medicine; Academic Press: Philadelphia, PA, USA, 2019; Volume 1. [Google Scholar]
- Titlbach, O.; Feyer, P.; Marek, H.; Kellner, K. Hormone changes as indicators of stress in the development and course of myo-cardial infarct. Z. Gesamte Inn. Med. 1989, 44, 82–87. [Google Scholar]
- Santos-Zas, I.; Lemarie, J.; Tedgui, A.; Ait-Oufella, H. Adaptive Immune Responses Contribute to Post-ischemic Cardiac Re-modeling. Front. Cardiovasc. Med. 2018, 5, 198. [Google Scholar] [CrossRef]
- Lapraz, J.-C.; Hedayat, K.M.; Pauly, P. Endobiogeny: A Global Approach to Systems Biology (Part 2 of 2). Glob. Adv. Health Med. 2013, 2, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, R.; Poeppinghaus, V.; Jones, G.; Peaston, M. Cortisol level predicts myocardial infarction in patients with ischaemic chest pain. Int. J. Cardiol. 1989, 25, 69–72. [Google Scholar] [CrossRef]
- Vetter, N.; Adams, W.; Strange, R.; Oliver, M. INITIAL METABOLIC AND HORMONAL RESPONSE TO ACUTE MYOCARDIAL INFARCTION. Lancet 1974, 303, 284–289. [Google Scholar] [CrossRef]
- Oliver, M.F. Metabolic response during impending myocardial infarction. II. Clinical implications. Circulation 1972, 45, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Selye, H. The Significance of the Adrenals for Adaptation. Science 1937, 85, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Walker, B.R.; Haw, S.; Newby, D.E.; Mackay, D.F.; Cobbe, S.M.; Pell, A.C.H.; Fischbacher, C.; Pringle, S.D.; Murdoch, D.; et al. Low serum cortisol predicts early death after acute myocardial infarction. Crit. Care Med. 2010, 38, 973–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, R.; Fox, J.; Jagger, J.; Davies, M.; Littler, W.; Murray, R. Serum cortisol levels predict infarct size and patient mortality. Int. J. Cardiol. 1992, 37, 145–150. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeneweg, F.L.; Karst, H.; De Kloet, E.R.; Joels, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011, 209, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Schuff, B.M.; Hedayat, K.M.; Lapraz, J.C.; Barsotti, T.; Golshan, S.; Hong, S.; Greenberg, B.H.; Mills, P.J.; Schuff, M.B.; Hedayat, M.K.; et al. A novel approach to modeling tissue-level activity of cortisol levels according to the theory of Endobiogeny, applied to chronic heart failure. J. Complex Health Sci. 2018, 1, 2–8. [Google Scholar] [CrossRef]
- Rao, M.S.; Devasia, T.; Kareem, H.; Padmakumar, R.; Ashwal, A.J. Serum Cortisol Level as a Predictor of In-Hospital Mortality in Patients Undergoing Primary Percutaneous Intervention for ST Segment Elevation Myocardial Infarction. Int. J. Cardiovasc. Pr. 2019, 4, 41–44. [Google Scholar] [CrossRef]
- Boonen, E.; Berghe, G.V.D. MECHANISMS IN ENDOCRINOLOGY: New concepts to further unravel adrenal insufficiency during critical illness. Eur. J. Endocrinol. 2016, 175, R1–R9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funder, J.W. Mineralocorticoid Receptors: Distribution and Activation. Heart Fail. Rev. 2005, 10, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Clore, J.; Schoolwerth, A.; Watlington, C.O. When is cortisol a mineralocorticoid? Kidney Int. 1992, 42, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.R. Organ-specific actions of 11 beta-hydroxysteroid dehydrogenase in humans: Implications for the pathophysi-ology of hypertension. Steroids 1994, 59, 84–89. [Google Scholar] [CrossRef]
- Park, J.; Choi, K.H.; Lee, J.M.; Kim, H.K.; Hwang, D.; Rhee, T.M.; Kim, J.; Park, T.K.; Yang, J.H.; Song, Y.B.; et al. Prognostic Implications of Door-to-Balloon Time and Onset-to-Door Time on Mortality in Patients with ST -Segment-Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e012188. [Google Scholar] [CrossRef] [Green Version]
- Shiomi, H.; Nakagawa, Y.; Morimoto, T.; Furukawa, Y.; Nakano, A.; Shirai, S.; Taniguchi, R.; Yamaji, K.; Nagao, K.; Suyama, T.; et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: Observational study. BMJ 2012, 344, e3257. [Google Scholar] [CrossRef] [Green Version]
- Alsamara, M.; Degheim, G.; Gholkar, G.; Hiner, E.; Zughaib, M. Is symptom to balloon time a better predictor of outcomes in acute ST-segment elevation myocardial infarction than door to balloon time? Am. J. Cardiovasc. Dis. 2018, 8, 43–47. [Google Scholar]
- Keller, T.; Zeller, T.; Ojeda, F.; Tzikas, S.; Lillpopp, L.; Sinning, C.; Wild, P.; Genth-Zotz, S.; Warnholtz, A.; Giannitsis, E.; et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011, 306, 2684–2693. [Google Scholar] [CrossRef]
- Fox, W.R.; Diercks, D.B. Troponin assay use in the emergency department for management of patients with potential acute coronary syndrome: Current use and future directions. Clin. Exp. Emerg. Med. 2016, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, V.S.; Jarolim, P. How to Interpret Elevated Cardiac Troponin Levels. Circulation 2011, 124, 2350–2354. [Google Scholar] [CrossRef] [Green Version]
- Morrow, D.A.; Cannon, C.P.; Jesse, R.L.; Newby, L.K.; Ravkilde, J.; Storrow, A.B.; Wu, A.H.; Christenson, R.H. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guide-lines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007, 115, e356–e375. [Google Scholar]
- Žaliaduonytė-Pekšienė, D.; Lesauskaitė, V.; Liutkevičienė, R.; Tamakauskas, V.; Kviesulaitis, V.; Šinkūnaitė-Maršalkienė, G.; Šimonytė, S.; Mačiulskytė, S.; Tamulevičiūtė-Prascienė, E.; Gustienė, O.; et al. Association of the genetic and traditional risk factors of ischaemic heart disease with STEMI and NSTEMI development. J. Renin Angiotensin Aldosterone Syst. 2017, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | Category | Number (Percentage) |
|---|---|---|
| Age, years M (SD) | 63.9, (SD ± 11.6) | |
| Gender, n, (%) | Male | 80 (65) |
| Female | 43 (35) | |
| Type of MI, n, (%) | STEMI | 79 (64) |
| Non STEMI | 44 (36) | |
| Type of MI, n, (%) | Anterior wall | 53 (43) |
| Posterior wall | 48 (39) | |
| Lateral wall | 22 (18) | |
| Comorbidities, n, (%) | Diabetes mellitus | 16 (13) |
| Hypertension | 112 (91) | |
| Dyslipidemia | 78 (63) | |
| CKD * | 5 (4) | |
| Old CVA | 12 (10) | |
| COPD | 5 (4) | |
| Killip classes, n, (%) | I | 48 (39) |
| II | 65 (53) | |
| III | 4 (3) | |
| IV | 6 (5) | |
| Onset to balloon time (in hours), n, (%) | <3 | 41 (33) |
| 4–6 | 22 (18) | |
| 7–12 | 21 (17) | |
| 13–48 | 38 (31) | |
| Ongoing pharmocological treatment, n, (%) | ACE inhibitors | 25 (20) |
| ARB | 7 (6) | |
| Nitrates | 7 (6) | |
| CCB | 12 (10) | |
| Beta blockers | 27 (22) | |
| Anticoagulant | 3 (2) | |
| Antiplatelet drugs | 7 (6) | |
| Statins | 3 (2) | |
| Diuretics | 9 (7) | |
| MRB | 1 (1) |
| Onset of Pain to Admission Time (in Hours) | p Value | ||||
|---|---|---|---|---|---|
| ≤3 | 4–6 | 7–12 | >13 | ||
| Median (25–75) | |||||
| Serum Cortisol 1 (nmol/L) | 895.1 (809.1–1002.0) ab | 778.7 (566.7–1157.7) c | 539.8 (350.3–846.9) a | 505.7 (388.1–713.9) bc | χ2 = 21.599; df = 3; p < 0.001 |
| Cortisol index 1 | 40.0 (14.0–131.2) ab | 190 (104.3–522.9) ac | 251.0 (93.0–504.7) bd | 76.6 (30.2–281.4) cd | χ2 = 19.296; df = 3; p < 0.001 |
| Cortisol index 2 | 15.4 (8.5–26.0) a | 17.6 (13.9–43.6) a | 17.2 (7.3–31.4) | 15.0 (7.3–38.1) | χ2 = 2.394; df = 3; p = 0.495 |
| Cortisol index 3 | 7.0 (4.7–14.6) a | 14.8 (8.1–21.3) a | 10.7 (7.3–15.7) | 12.2 (5.8–24.1) | χ2 = 4.833; df = 3; p = 0.184 |
| ∆Cortisol index 1–2 | 22.8 (1.4–89.3) ab | 176.7 (99.8–346.7) ac | 198.9 (58.5–485.6) bd | 39.3 (10.8–139.9) cd | χ2 = 17.3; df = 3; p = 0.001 |
| ∆Cortisol index 1–3 | 27.5 (5.4–140.0) a | 218.0 (97.5–530.1) ac | 159.3 (55.4–492.9) | 36.4 (7.3–169.7) c | χ2 = 10.44; df = 3; p = 0.015 |
| Serum Cortisol 1 (nmol/L) | Cortisol Index 1 | Cortisol Index 2 | Cortisol Index 3 | |
|---|---|---|---|---|
| N | 94 | 122 | 83 | 75 |
| M (SD) | 753.1 (351.8) | 242.5 (342) | 26.4 (32) | 16.3 (20) |
| Median (25–75%) | 778.5 (466.6–955.4) | 102.6 (38.7–291.9) ab | 15.4 (8.7–31.9) ac | 10.7 (5.9–19.3) bc |
| Troponin I 1 | Troponin I Peak | |
|---|---|---|
| Serum cortisol 1 | r = −0.410; p = 0.001 | r = 0.376; p = 0.001 |
| Cortisol index 1 | r = −0.007; p = 0.935 | r = 0.163; p = 0.073 |
| ∆Cortisol index 1–2 | r = 0.358; p = 0.001 | r = 0.247; p = 0.023 |
| ∆Cortisol index 1–3 | r = 0.195; p = 0.097 | r = 0.233; p = 0.046 |
| Parameters | Survivors Group n = 111/24 | Non-Survivors Group n = 12 | p Value |
|---|---|---|---|
| Median (25–75%) | |||
| Serum cortisol 1 | 817.7 (476.3–978.2) 887.3 (558.6–971.5) * | 595.4 (416–722.2) | 0.039 0.02 * |
| Cortisol index 1 | 89 (35.5–261.6) 75.9 (13.7–276) * | 253.0 (104.2–454.2) | 0.043 0.048 * |
| Troponin 1 | 1.08 (0.1–3.96) 1.03 (0.07–2.67) * | 4.36 (1.58–11.53) | 0.018 0.012 * |
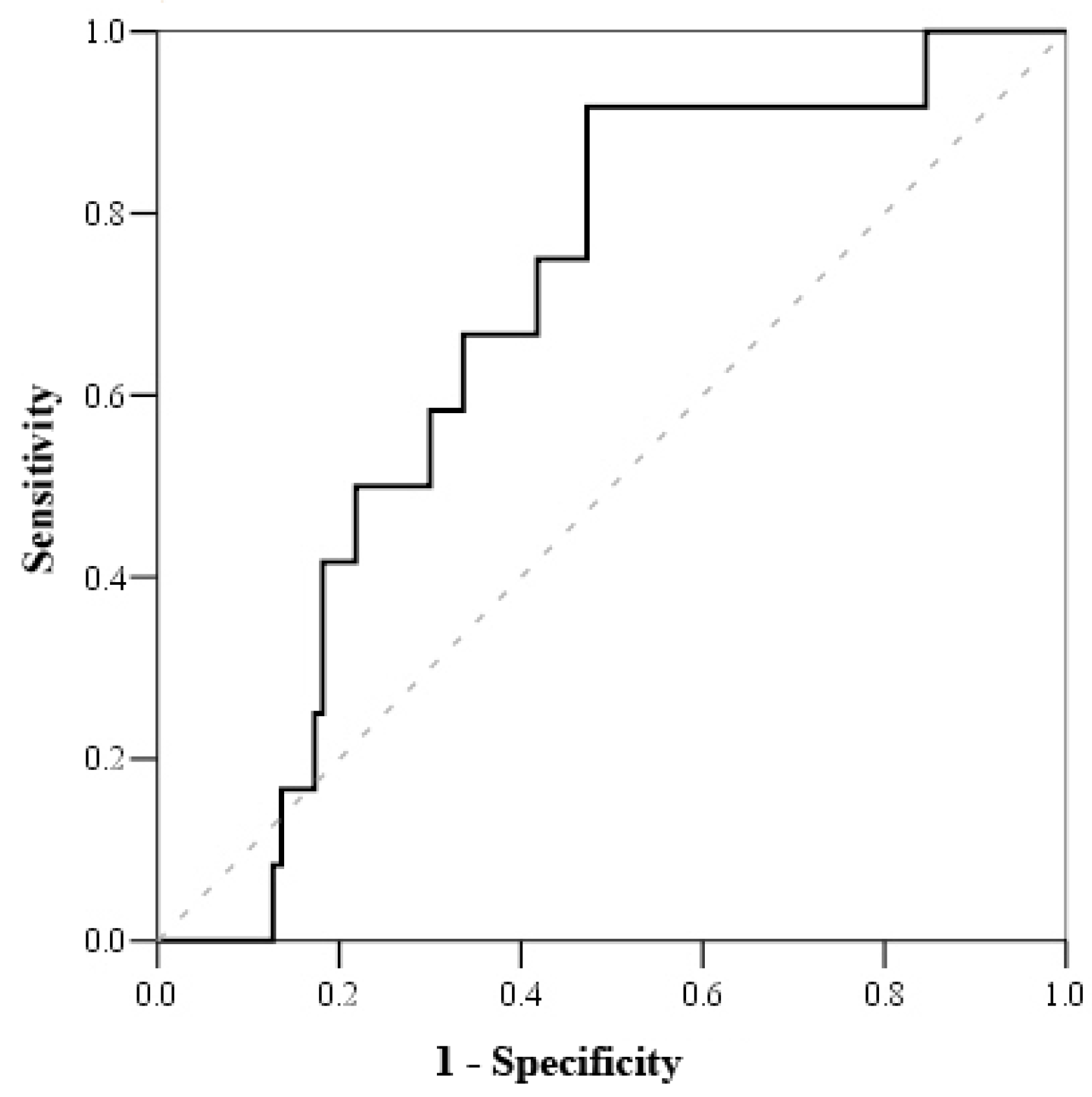
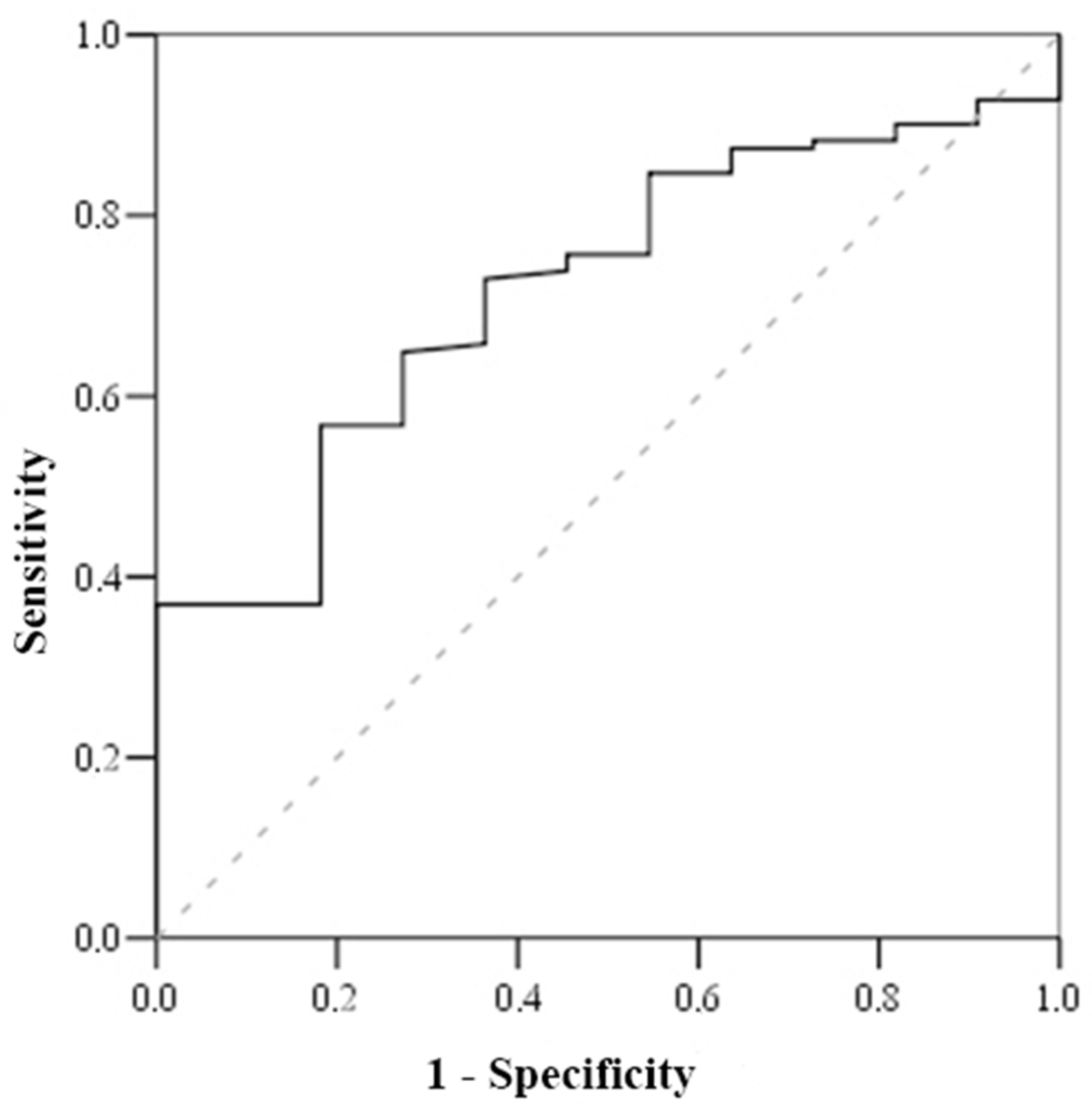
| Parameters/ Optimal Meaning of Its Change | AUC-ROC Curve (%) | Sensitivity/Specificity | Henly-McNeil Method z Score p Value | Survival/Death (%) | p Value | Death RR (95% CI) |
|---|---|---|---|---|---|---|
| Serum cortisol 1 <800 nmol/L | 70.0 | 100 51.2 | 3.27 0.001 | 0 19.6 | 0.002 | - |
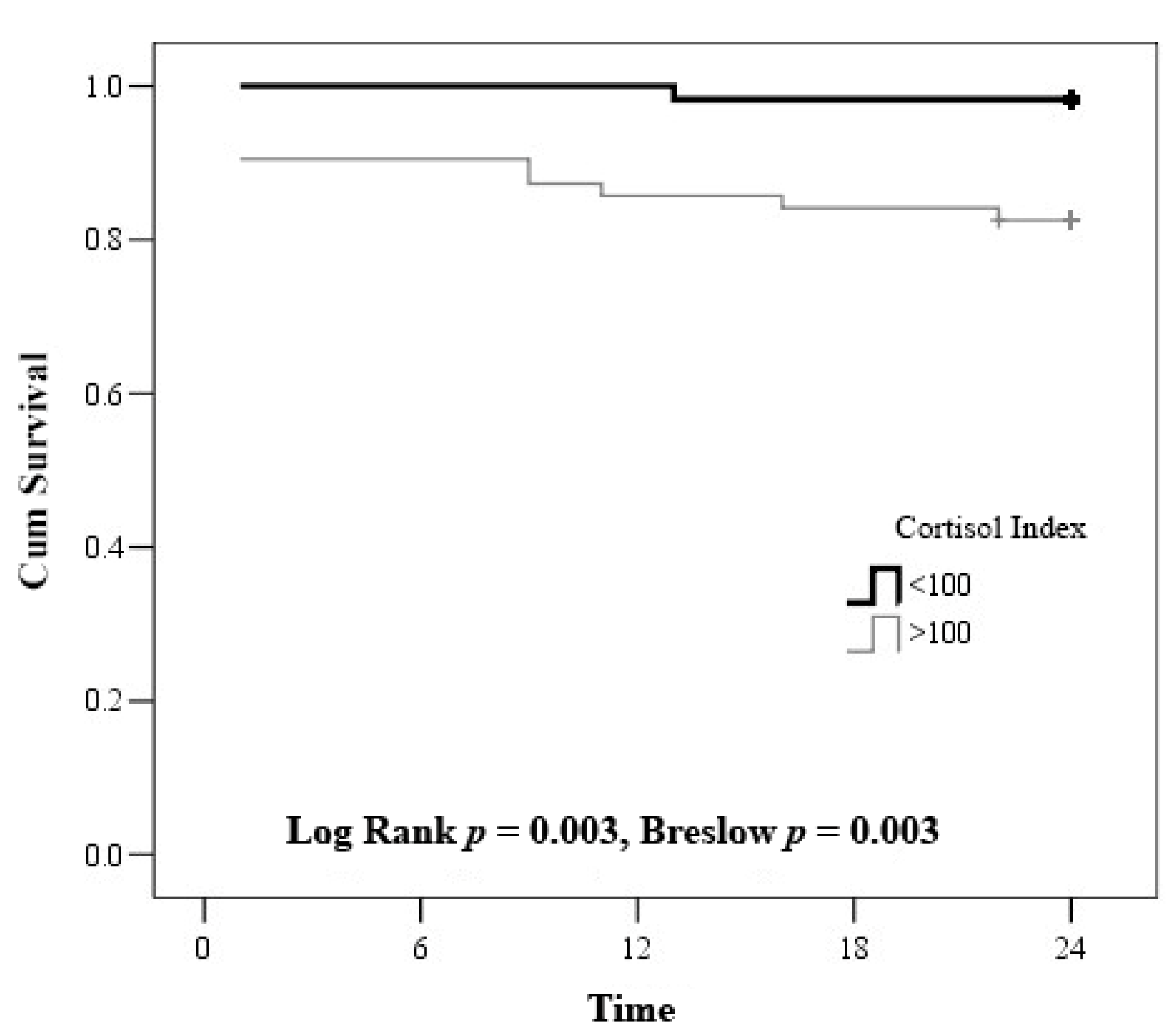
| Cortisol index 1 >100 | 78.0 | 83.3 52.7 | 2.7 0.007 | 3.3 15.8 | 0.018 | 5.179 (1.134–23.65) |
| Troponin I 1 > 1.56 μg/L | 71.7 | 81.8 56.8 | 3.3 0.001 | 3.1 15.8 | 0.014 | 5.326 (1.15–24.655) |
| Troponin I > 1.56 μmol/L and Cortisol Index > 100 μmol/L (n = 35) | Troponin I < 1.56 μmol/L and Cortisol Index > 100 (n = 26) | Troponin I > 1.56 μmol/L and Cortisol Index < 100 (n = 22) | Troponin < 1.56 μmol/L and Cortisol Index < 100 (n = 38) | |
|---|---|---|---|---|
| n (%) | ||||
| Non-survivors group | 7(20.0) * | 2(7.7) | 2(9.1) | 0 |
| 4(4.7) * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braukyliene, R.; Hedayat, K.; Zajanckauskiene, L.; Jurenas, M.; Unikas, R.; Aldujeli, A.; Petrokas, O.; Zabiela, V.; Steponaviciute, R.; Vitkauskiene, A.; et al. Prognostic Value of Cortisol Index of Endobiogeny in Acute Myocardial Infarction Patients. Medicina 2021, 57, 602. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57060602
Braukyliene R, Hedayat K, Zajanckauskiene L, Jurenas M, Unikas R, Aldujeli A, Petrokas O, Zabiela V, Steponaviciute R, Vitkauskiene A, et al. Prognostic Value of Cortisol Index of Endobiogeny in Acute Myocardial Infarction Patients. Medicina. 2021; 57(6):602. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57060602
Chicago/Turabian StyleBraukyliene, Rima, Kamyar Hedayat, Laura Zajanckauskiene, Martynas Jurenas, Ramunas Unikas, Ali Aldujeli, Osvaldas Petrokas, Vytautas Zabiela, Rasa Steponaviciute, Astra Vitkauskiene, and et al. 2021. "Prognostic Value of Cortisol Index of Endobiogeny in Acute Myocardial Infarction Patients" Medicina 57, no. 6: 602. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57060602








