Neutrophil-to-Lymphocyte Ratio as an Independent Predictor of In-Hospital Mortality in Patients with Acute Intracerebral Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. NLR Assessed at Admission
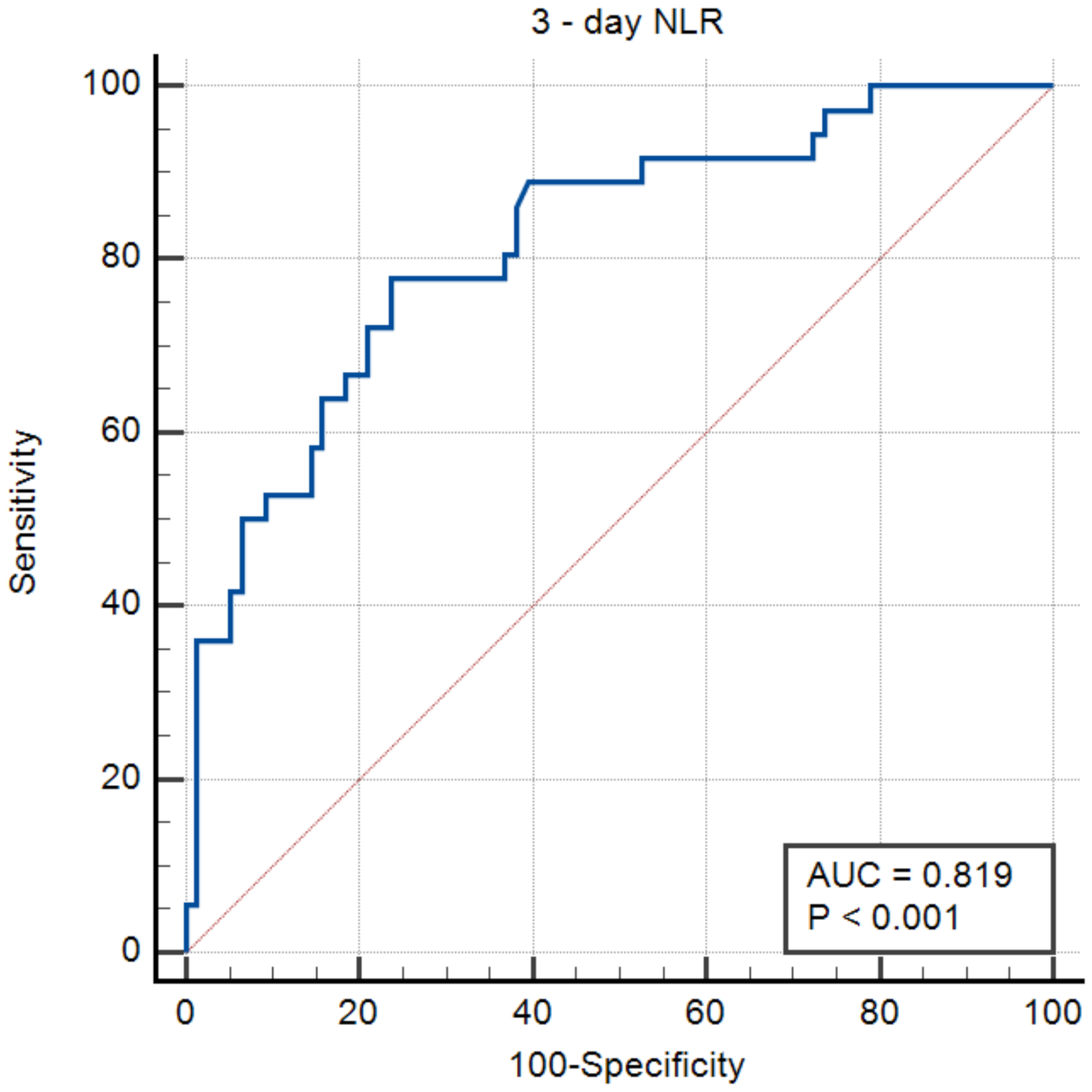
3.2. NLR Assessed 72 h after Admission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Al-Kawaz, M.N.; Hanley, D.F.; Ziai, W. Advances in Therapeutic Approaches for Spontaneous Intracerebral Hemorrhage. Neurotherapeutics 2020, 17, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Claude, H.J.; David, C.B.; Lavrentios, B.; Geoffrey, T.M.; Claiborne, J.S. The ICH Score. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Al-Shahi Salman, R.; Frantzias, J.; Lee, R.J.; Lyden, P.D.; Battey, T.W.K.; Ayres, A.M.; Goldstein, J.N.; Mayer, S.A.; Steiner, T.; Wang, X.; et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: A systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018, 17, 885–894. [Google Scholar] [CrossRef] [Green Version]
- Simona, S.; Carmine, M.; Danilo, T.; Luigi, O.; Antonio, C. Incidence and 10-Year Survival of Intracerebral Hemorrhage in a Population-Based Registry. Stroke 2009, 40, 394–399. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.J.; Price, C.I. A Systematic Review and Meta-Analysis of Molecular Biomarkers Associated with Early Neurological Deterioration Following Acute Stroke. Cerebrovasc. Dis. 2018, 46, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. stroke 2020, 22, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Hu, S.; Ding, Y.; Ju, X.; Wang, L.; Lu, Q.; Wu, X. Neutrophil-to-Lymphocyte Ratio and 30-Day Mortality in Patients with Acute Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2016, 25, 182–187. [Google Scholar] [CrossRef]
- Giede-Jeppe, A.; Bobinger, T.; Gerner, S.T.; Sembill, J.A.; Sprügel, M.I.; Beuscher, V.D.; Lücking, H.; Hoelter, P.; Kuramatsu, J.B.; Huttner, H.B. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor for In-Hospital Mortality in Spontaneous Intracerebral Hemorrhage. Cerebrovasc. Dis. 2017, 44, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, X.; Chen, S.; Xiao, Y.; Zhuang, W. Neutrophil–lymphocyte ratio predicts the outcome of intracerebral hemorrhage: A meta-analysis. Medicine 2019, 98, e16211. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, F.; Quan, Y.; Wang, L.; Xia, J.-J.; Jiang, T.-T.; Shen, L.-J.; Kang, W.-H.; Ding, Y.; Mei, L.-X.; et al. Early increase of neutrophil-to-lymphocyte ratio predicts 30-day mortality in patients with spontaneous intracerebral hemorrhage. CNS Neurosci. Ther. 2019, 25, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radu, R.A.; Terecoasa, E.O.; Tiu, C.; Ghita, C.; Purcaru, L.I.; Marinescu, A.N.; Bajenaru, O.A. Clinical Characteristics and Outcomes of Patients with Intracerebral Hemorrhage—A Feasibility Study on Romanian Patients. J. Med. Life 2020, 13, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Schmitt, A.; Wilson, D.; Yakushiji, Y.; Gregoire, S.M.; Fox, Z.; Jäger, H.R.; Werring, D.J. The Cerebral Haemorrhage Anatomical RaTing inStrument (CHARTS): Development and assessment of reliability. J. Neurol. Sci. 2017, 372, 178–183. [Google Scholar] [CrossRef]
- Kothari, R.U.; Brott, T.; Broderick, J.P.; Barsan, W.G.; Sauerbeck, L.R.; Zuccarello, M.; Khoury, J. The ABCs of Measuring Intracerebral Hemorrhage Volumes. Stroke 1996, 27, 1304–1305. [Google Scholar] [CrossRef]
- Graeb, D.A.; Robertson, W.D.; Lapointe, J.S.; Nugent, R.A.; Harrison, P.B. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 1982, 143, 91–96. [Google Scholar] [CrossRef]
- Delcea, C.; Buzea, C.A.; Dan, G.A. The neutrophil to lymphocyte ratio in heart failure: A comprehensive review. Rom. J. Intern. Med. 2019, 57, 296–314. [Google Scholar] [CrossRef] [Green Version]
- Ham, S.Y.; Yoon, H.J.; Nam, S.B.; Yun, B.H.; Eum, D.; Shin, C.S. Prognostic value of neutrophil/lymphocyte ratio and mean platelet volume/platelet ratio for 1-year mortality in critically ill patients. Sci. Rep. 2020, 10, 21513. [Google Scholar] [CrossRef]
- Mei, S.; Shao, Y.; Fang, Y.; Lu, J.; Zheng, J.; Xu, S.; Wu, H.; Sun, Z.; Yu, J.; Chen, S.; et al. The Changes of Leukocytes in Brain and Blood After Intracerebral Hemorrhage. Front. Immunol. 2021, 12, 261. [Google Scholar] [CrossRef]
- Shi, K.; Wood, K.; Shi, F.-D.; Wang, X.; Liu, Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc. Neurol. 2018, 3, 34–41. [Google Scholar] [CrossRef]
- Béjot, Y.; Grelat, M.; Delpont, B.; Durier, J.; Rouaud, O.; Osseby, G.-V.; Hervieu-Bègue, M.; Giroud, M.; Cordonnier, C. Temporal trends in early case-fatality rates in patients with intracerebral hemorrhage. Neurology 2017, 88, 985–990. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, S.; Castillo-Lorente, E.; Guerrero-Lopez, F.; Rodríguez-Rubio, D.; Aguilar-Alonso, E.; Lafuente-Baraza, J.; Gómez-Jiménez, F.J.; Mora-Ordóñez, J.; Rivera-López, R.; Arias-Verdú, M.D.; et al. Validation of the ICH score in patients with spontaneous intracerebral haemorrhage admitted to the intensive care unit in Southern Spain. BMJ Open 2018, 8, e021719. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Gurol, M.E.; Huang, Z.; Lichtenstein, A.H.; Wang, X.; Wang, Y.; Neumann, S.; Wu, S.; Gao, X. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage. Neurology 2019, 93, e445–e457. [Google Scholar] [CrossRef]
- David, R.-L.; Marta, R.; Marc, R.; Pilar, C.; Jorge, P.; Socorro, P.; Bernardo, I.; Pilar, M.; Olga, M.; Francisco, R.; et al. Serum Low-Density Lipoprotein Cholesterol Level Predicts Hematoma Growth and Clinical Outcome After Acute Intracerebral Hemorrhage. Stroke 2011, 42, 2447–2452. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.N.; Leasure, A.C.; Kuohn, L.R.; Both, C.P.; Petersen, N.H.; Sansing, L.H.; Matouk, C.C.; Testai, F.; Langefeld, C.D.; Woo, D.; et al. Admission Hemoglobin Levels Are Associated With Functional Outcome in Spontaneous Intracerebral Hemorrhage. Crit. Care Med. 2021, 49, 828–837. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Jiang, T.; Xia, J.; Xu, F.; Shen, L.; Kang, W.; Ding, Y.; Mei, L.; Ju, X.; et al. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor of 30-Day Mortality of Intracerebral Hemorrhage Patients: A Validation Cohort Study. Neurotox. Res. 2018, 34, 347–352. [Google Scholar] [CrossRef] [Green Version]

| Parameter (Unit) | Overall n = 201 (100%) | Alive at Discharge n = 111 (55.2%) | In-Hospital Death n = 90 (44.8%) | p |
|---|---|---|---|---|
| Age (median, 25–75 IQR) | 70 (61–79) | 66 (56–75) | 73 (63–81.25) | 0.001 |
| Gender (n, % male) | 111 (55.2%) | 60 (54.1%) | 51 (45.9%) | 0.7 |
| Smokers | 33 (16.4%) | 17 (15.3%) | 17 (18.9%) | 0.5 |
| Chronic alcohol intake | 48 (23.8%) | 28 (25.2%) | 20 (22.2%) | 0.6 |
| Medical History (n, %) | ||||
| Previous Ischemic Stroke | 7 (3.5%) | 4 (3.6%) | 3 (3.3%) | 0.9 |
| Previous Hemorrhagic Stroke | 27 (13.4%) | 18 (16.2%) | 9 (10%) | 0.2 |
| Previous Known Hypertension | 154 (76.5%) | 89 (80%) | 65 (72%) | 0.2 |
| Diabetes mellitus | 50 (25%) | 27 (24.3%) | 43 (47.8%) | <0.001 |
| Dyslipidemia | 122 (60.7%) | 82 (73.8%) | 40 (44.4%) | <0.001 |
| Atrial fibrillation | 27 (13.47%) | 13 (11.8%) | 14 (15.6%) | 0.4 |
| Coronary artery disease | 17 (8.5%) | 4 (3.6%) | 13 (14.4%) | 0.009 |
| Previous treatment (n, %) | ||||
| Previous antiplatelet treatment | 52 (25.8%) | 29 (26.1%) | 23 (25.6%) | 0.9 |
| Previous anticoagulant treatment | 25 (12.44%) | 10 (9.01%) | 15 (16.7%) | 0.1 |
| Previous antihypertensive treatment | 186 (51%) | 54 (48.6%) | 49 (54.4%) | 0.3 |
| Clinical ICH severity (median, 25–75 IQR) | ||||
| ICH score | 1 (0–2) | 1 (0–1) | 2 (1–3) | <0.001 |
| Admission NIHSS | 16 (6–24) | 10 (4–17) | 25 (16–36) | <0.001 |
| Admission GCS | 15 (10–15) | 15 (15–15) | 9.5 (3–14) | <0.001 |
| Pre-Stroke mRS | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.4 |
| ICH imaging | ||||
| Hematoma Volume (mL, 25–75 IQR) | 13.7 (4.6–35.2) | 7.3 (1.8–19.9) | 27.8 (11.2–63.7) | <0.001 |
| HV < 10 mL | 80 (41%) | 62 (57.4%) | 18 (20.7%) | <0.001 |
| HV 10–29 mL | 58 (29.7%) | 30 (27.8%) | 28 (32.2%) | |
| HV 30–49 mL | 27 (13.8%) | 11 (10.2%) | 16 (18.4%) | |
| HV ≥ 50 mL | 30 (15.3%) | 5 (4.6%) | 25 (28.7%) | |
| Lobar hemorrhage (n, %) | 50 (24.8%) | 25 (22.5%) | 25 (27.8%) | 0.4 |
| Deep hemorrhage (n, %) | 140 (69.6%) | 81 (72.9%) | 59 (65.5%) | 0.2 |
| Ventricular effraction (n, %) | 90 (44.9%) | 35 (31.2%) | 56 (62.1%) | <0.001 |
| GRAEB score (median, 25–75 IQR) | 0 (0–4) | 0 (0–1) | 2 (0–8) | <0.001 |
| Admission Blood Analyses (median, 25–75 IQR) | ||||
| Creatinine Clearance (mL/min/1.73 m2) | 79 (55.3–91.4) | 80.8 (59.8–93.1) | 73.5 (49.4–89.5) | 0.07 |
| Glucose (mg/dL) | 141 (110.5–191.5) | 126 (104–153) | 176.5 (132–217) | <0.001 |
| Glucose > 180 mg/dL (n, %) | 56 (27.8%) | 16 (14.4%) | 40 (44.4%) | <0.001 |
| WBC (cells/mm3) | 10,040 (7900–12,950) | 9400 (7820–11,400) | 12,130 (8310–14,922) | <0.001 |
| NEUT (cells/mm3) | 7200 (5050–10,250) | 6600 (4900–8600) | 9145 (5750–12,500) | <0.001 |
| LYMPHs (cells/mm3) | 1600 (1105–2300) | 1700 (1200–2400) | 1550 (1000–2300) | 0.4 |
| MONOs (cells/mm3) | 600 (400–885) | 600 (400–800) | 605 (475–900) | 0.1 |
| HGB (g/dL) | 13.8 (12.5–14.9) | 13.9 (12.6–15.4) | 13.4 (12.2–14.7) | 0.05 |
| PLTs (cells × 1000/mm3) | 209 (162–268) | 213 (167–267) | 208 (160–270) | 0.9 |
| INR | 1.1 (1.04–1.2) | 1.1 (1.04–1.18) | 1.1 (1.06–1.39) | 0.01 |
| Admission NLR | 4.3 (2.6–7.8) | 3.7 (2.4–6) | 5.6 (2.9–9.8) | 0.002 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| Age | 0.001 | 1.03 (1.01–1.06) | <0.001 | 1.07 (1.03–1.11) |
| Admission NIHSS | <0.001 | 1.15 (1.1–1.2) | <0.001 | 1.13 (1.07–1.19) |
| Coronary artery disease | 0.009 | 4.5 (1.4–14.3) | 0.06 | 4.6 (0.9–23.5) |
| Admission HV | <0.001 | 1.03 (1.02–1.05) | 0.02 | 1.02 (1.01–1.05) |
| Admission GRAEB score | <0.001 | 1.2 (1.1–1.4) | 0.1 | 1.1 (0.9–1.2) |
| Creatinine Clearance | 0.07 | 0.99 (0.97–1) | 0.7 | 1.01 (0.9–1.01) |
| Glucose >180 mg/dL | <0.001 | 4.7 (2.3–8.7) | 0.006 | 3.9 (1.4–10.5) |
| Admission NLR | 0.002 | 1.1 (1.04–1.18) | 0.3 | 1.04 (0.9–1.1) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| Age | 0.01 | 1.05 (1.02–1.08) | 0.02 | 1.05 (1.007–1.1) |
| Admission NIHSS | <0.001 | 1.12 (1.07–1.2) | 0.06 | 1.06 (0.9–1.1) |
| Admission HV | <0.001 | 1.03 (1.01–1.04) | 0.07 | 1.02 (0.9–1.04) |
| Three-day NLR | <0.001 | 1.3 (1.1–1.4) | <0.001 | 1.2 (1.09–1.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, R.A.; Terecoasă, E.O.; Tiu, C.; Ghiță, C.; Nicula, A.I.; Marinescu, A.N.; Popescu, B.O. Neutrophil-to-Lymphocyte Ratio as an Independent Predictor of In-Hospital Mortality in Patients with Acute Intracerebral Hemorrhage. Medicina 2021, 57, 622. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57060622
Radu RA, Terecoasă EO, Tiu C, Ghiță C, Nicula AI, Marinescu AN, Popescu BO. Neutrophil-to-Lymphocyte Ratio as an Independent Predictor of In-Hospital Mortality in Patients with Acute Intracerebral Hemorrhage. Medicina. 2021; 57(6):622. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57060622
Chicago/Turabian StyleRadu, Răzvan Alexandru, Elena Oana Terecoasă, Cristina Tiu, Cristina Ghiță, Alina Ioana Nicula, Andreea Nicoleta Marinescu, and Bogdan Ovidiu Popescu. 2021. "Neutrophil-to-Lymphocyte Ratio as an Independent Predictor of In-Hospital Mortality in Patients with Acute Intracerebral Hemorrhage" Medicina 57, no. 6: 622. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57060622






