Antimicrobial Activity of Chitosan Oligosaccharides with Special Attention to Antiparasitic Potential
Abstract
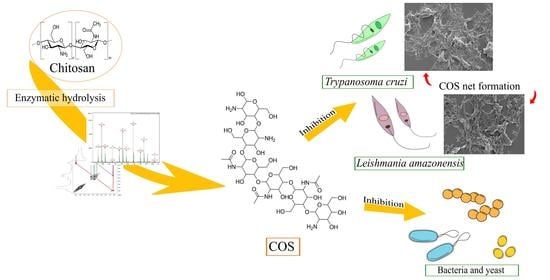
:1. Introduction
2. Results
2.1. COS Production and Analysis
2.1.1. Nuclear Magnetic Resonance Spectroscopy
2.1.2. Mass Spectrometry
2.2. Biocompatibility Assay
2.3. The Antimicrobial Activity of COSs
2.4. Antiparasitic Activity of COSs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chitosanase Production
4.3. COS Production
4.4. COS Characterization
4.5. Biocompatibility Assay
4.6. In Vitro Antimicrobial Activity of COSs
4.7. In Vitro Antiparasitic Activity of COSs
4.7.1. Leishmania Amazonensis
4.7.2. Trypanosoma Cruzi
4.7.3. Morphological Analysis Using Scanning Electron Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendation; Government of the United Kingdom: London, UK, 2016.
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [Green Version]
- Filho, W.L.; Scheday, S.; Boenecke, J.; Gogoi, A.; Maharaj, A.; Korovou, S. Climate change, health and mosquito-borne diseases: Trends and implications to the pacific region. Int. J. Environ. Res. Public Health 2019, 16, 5114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, L.K.; Kochmann, J.; Klimpel, S.; Cunze, S. Modeling the climatic suitability of leishmaniasis vector species in Europe. Sci. Rep. 2017, 7, 13325. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.D.; Guhl, F.; Vallejo, G.A.; Ramírez, J.D. The effect of temperature increase on the development of Rhodnius prolixus and the course of Trypanosoma cruzi metacyclogenesis. PLoS Negl. Trop. Dis. 2018, 12, e0006735. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Weiss, L.M.; Montgomery, S.P. Chagas disease has now gone global. PLoS Negl. Trop. Dis. 2011, 5, e1136. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; López-Vélez, R. Challenges in the management of Chagas disease in Latin-American migrants in Europe. Clin. Microbiol. Infect. 2017, 23, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukeš, J.; Butenko, A.; Hashimi, H.; Maslov, D.A.; Votýpka, J.; Yurchenko, V. Trypanosomatids Are Much More than Just Trypanosomes: Clues from the Expanded Family Tree. Trends Parasitol. 2018, 34, 466–480. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Fourth WHO Report on Neglected Tropical Diseases: Integrating Neglected Tropical Diseases into Global Health and Development; World Health Organization: Geneva, Switzerland, 2017; Volume 4. [Google Scholar]
- PAHO. Pan American Health Organization Leishmaniasis: Epidemiological Report in the Americas; PAHO: Washington, DC, USA, 2019; pp. 2–5. [Google Scholar]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2017, 184, 38–52. [Google Scholar] [CrossRef]
- Bermudez, J.; Davies, C.; Simonazzi, A.; Pablo Real, J.; Palma, S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discov. Today 2017, 22, 1516–1531. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Don’t waste seafood waste: Turning cast-off shells into nitrogen-rich chemicals would benefit economies and the environment. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of antibacterial bacterial cellulose composite membranes modified with chitosan or chitooligosaccharide. Carbohydr. Polym. 2020, 229, 115520. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.-S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Fernández, M.; Alemany, S.; Heras, A.; Acosta, N. Influence of Preparation Methods of Chitooligosaccharides on Their Physicochemical Properties and Their Anti-Inflammatory Effects in Mice and in RAW264.7 Macrophages. Mar. Drugs 2018, 16, 430. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Yuan, S.; Yang, X.; Guo, Y.; Li, L.; Xu, C.; Zhai, X.; Wang, J. Structural characterization and antitumor effects of chitosan oligosaccharides against orthotopic liver tumor via NF-κB signaling pathway. J. Funct. Foods 2019, 57, 157–165. [Google Scholar] [CrossRef]
- El-Sayed, S.T.; Omar, N.I.; El-Sayed, E.S.M.; Shousha, W.G. Evaluation Antioxidant and cytotoxic activities of novel chitooligosaccharides prepared from chitosan via enzymatic hydrolysis and ultrafiltration. J. Appl. Pharm. Sci. 2017, 7, 50–55. [Google Scholar]
- Zhu, L.; Li, R.; Jiao, S.; Wei, J.; Yan, Y.; Wang, Z.A.; Li, J.; Du, Y. Blood-brain barrier permeable chitosan oligosaccharides interfere with β-Amyloid aggregation and alleviate β-amyloid protein mediated neurotoxicity and neuroinflammation in a dose-and degree of polymerization-dependent manner. Mar. Drugs 2020, 18, 488. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Marmouzi, I.; Ezzat, S.M.; Salama, M.M.; Merghany, R.M.; Attar, A.M.; EL-Desoky, A.M.; Mohamed, S.O. Recent Updates in Pharmacological Properties of Chitooligosaccharides. Biomed. Res. Int. 2019, 2019, 4568039. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Xu, Z.; Shi, Y.; Lin, C.; Jiao, S.; Zhang, L.; Li, P. Multivalent and synergistic chitosan oligosaccharide-Ag nanocomposites for therapy of bacterial infection. Sci. Rep. 2020, 10, 10011. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Tian, M.; Tan, H.; Li, H.; You, C. Molecular weight dependence of structure and properties of chitosan oligomers. RSC Adv. 2015, 5, 69445–69452. [Google Scholar] [CrossRef]
- De Medeiros Dantas, J.M.; da Silva, N.S.; de Padilha, C.E.A.; de Araújo, N.K.; dos Santos, E.S. Enhancing chitosan hydrolysis aiming chitooligosaccharides production by using immobilized chitosanolytic enzymes. Biocatal. Agric. Biotechnol. 2020, 28, 101759. [Google Scholar] [CrossRef]
- Riezk, A.; Raynes, J.G.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of chitosan and its derivatives against Leishmania major and Leishmania mexicana in vitro. Antimicrob. Agents Chemother. 2020, 64, e01772-19. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, N.K.; Pagnoncelli, M.G.B.; Pimentel, V.C.; Xavier, M.L.O.; Padilha, C.E.A.; de Macedo, G.R.; dos Santos, E.S. Single-step purification of chitosanases from Bacillus cereus using expanded bed chromatography. Int. J. Biol. Macromol. 2016, 82, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z. Determination of the deacetylation degree of chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef]
- Liu, F.C.; Su, C.R.; Wu, T.Y.; Su, S.G.; Yang, H.L.; Lin, J.H.Y.; Wu, T.S. Efficient 1H-NMR quantitation and investigation of N-Acetyl-d-glucosamine (GlcNAc) and N,N′-diacetylchitobiose (GlcNAc)2 from chitin. Int. J. Mol. Sci. 2011, 12, 5828–5843. [Google Scholar] [CrossRef]
- Fukamizo, T.; Hayashi, K. Separation and mutarotation of anomers of chitooligosaccharides. J. Biochem. 1982, 91, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Fukamizo, T.; Goto, S.; Ohtakara, A.; Mitsutomi, M. NMR Spectra of Partially Deacetylated Chitotrisaccharides. Agric. Biol. Chem. 1991, 55, 2653–2655. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.; Hwang, J.; Kim, S.; Jeon, Y.; Je, J.; Ahn, C.; Moon, S.; Jeon, B.; Park, P. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.G.; Li, X.; Yu, L.S.; Liang, J.; Sun, H.M.; Kuang, H.X. Structural-fingerprinting of polysaccharides to discern Panax species by means of gas-liquid chromatography and mass spectrometry. Int. J. Biol. Macromol. 2020, 151, 932–943. [Google Scholar] [CrossRef]
- Li, P.; Linhardt, R.J.; Cao, Z. Structural characterization of oligochitosan elicitor from Fusarium sambucinum and its elicitation of defensive responses in Zanthoxylum bungeanum. Int. J. Mol. Sci. 2016, 17, 2076. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Wu, H.; Wei, P.; Pan, M.; Tuo, Y.; Kusakabe, I.; Du, Y. Potent angiogenic inhibition effects of deacetylated chitohexaose separated from chitooligosaccharides and its mechanism of action in vitro. Carbohydr. Res. 2009, 344, 1975–1983. [Google Scholar] [CrossRef]
- De Assis, C.F.; Costa, L.S.; Melo-Silveira, R.F.; Oliveira, R.M.; Pagnoncelli, M.G.B.; Rocha, H.A.O.; de Macedo, G.R.; dos Santos, E.S. Chitooligosaccharides antagonize the cytotoxic effect of glucosamine. World J. Microbiol. Biotechnol. 2012, 28, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.N.; Mei, L.H.I.; Santos, A.R. Sorbitol-plasticized and neutralized chitosan membranes as skin substitutes. Mater. Res. 2015, 18, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Santos-Moriano, P.; Kidibule, P.; Míguez, N.; Fernández-Arrojo, L.; Ballesteros, A.O.; Fernández-Lobato, M.; Plou, F.J. Tailored enzymatic synthesis of chitooligosaccharides with different deacetylation degrees and their anti-inflammatory activity. Catalysts 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.T.; Chen, T.L.; Chen, R.M. Lipopolysaccharide triggers macrophage activation of inflammatory cytokine expression, chemotaxis, phagocytosis, and oxidative ability via a toll-like receptor 4-dependent pathway: Validated by RNA interference. Toxicol. Lett. 2009, 191, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Li, P. Size and pH effects of chitooligomers on antibacterial activity against Staphylococcus aureus. Int. J. Biol. Macromol. 2014, 64, 302–305. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, Ó.S.; João Monteiro, M.; Pintado, M.E.; Xavier Malcata, F. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef]
- Khan, F.; Lee, J.W.; Manivasagan, P.; Pham, D.T.N.; Oh, J.; Kim, Y.M. Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microb. Pathog. 2019, 135, 103623. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Byun, H.G.; Moon, S.H.; Kim, S.K. Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights. J. Microbiol. Biotechnol. 2004, 14, 317–323. [Google Scholar]
- Zhang, G.; Li, X.; Xu, X.; Tang, K.; Vu, V.H.; Gao, P.; Chen, H.; Xiong, Y.L.; Sun, Q. Antimicrobial activities of irradiation-degraded chitosan fragments. Food Biosci. 2019, 29, 94–101. [Google Scholar] [CrossRef]
- Pavinatto, A.; Delezuk, J.A.M.; Souza, A.L.; Pavinatto, F.J.; Volpati, D.; Miranda, P.B.; Campana-Filho, S.P.; Oliveira, O.N. Experimental evidence for the mode of action based on electrostatic and hydrophobic forces to explain interaction between chitosans and phospholipid Langmuir monolayers. Colloids Surf. B Biointerfaces 2016, 145, 201–207. [Google Scholar] [CrossRef]
- Husain, T.; Baig, M.M.F.A.; Kakar, M.U.; Ihsan, A.U.; Zhou, Q.-G.; Shumzaid, M.; Akabar, M.D.; Sohail, M.; Naveed, M.; Phil, L.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar]
- Li, R.; Yuan, X.; Wei, J.; Zhang, X.; Cheng, G.; Wang, Z.A.; Du, Y. Synthesis and Evaluation of a Chitosan Oligosaccharide-Streptomycin Conjugate against Pseudomonas aeruginosa Biofilms. Mar. Drugs 2019, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight-activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef]
- Palmeira-De-Oliveira, A.; Ribeiro, M.P.; Palmeira-De-Oliveira, R.; Gaspar, C.; Costa-De-Oliveira, S.; Correia, I.J.; Pina Vaz, C.; Martinez-De-Oliveira, J.; Queiroz, J.A.; Rodrigues, A.G. Anti-Candida activity of a chitosan hydrogel: Mechanism of action and cytotoxicity profile. Gynecol. Obstet. Investig. 2010, 70, 322–327. [Google Scholar] [CrossRef]
- Mei, Y.X.; Dai, X.Y.; Yang, W.; Xu, X.W.; Liang, Y.X. Antifungal activity of chitooligosaccharides against the dermatophyte Trichophyton rubrum. Int. J. Biol. Macromol. 2015, 77, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Koo, J.C.; Park, J.K. Antifungal Effect of Chitosan as Ca2+ Channel Blocker. Plant Pathol. J. 2016, 32, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Galván Márquez, I.; Akuaku, J.; Cruz, I.; Cheetham, J.; Golshani, A.; Smith, M.L. Disruption of protein synthesis as antifungal mode of action by chitosan. Int. J. Food Microbiol. 2013, 164, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Shovan, L.R.; Hjeljord, L.G.; Aam, B.B.; Eijsink, V.G.H.; Sørlie, M.; Tronsmo, A. Inhibition of fungal plant pathogens by synergistic action of chito-oligosaccharides and commercially available fungicides. PLoS ONE 2014, 9, e93192. [Google Scholar]
- Ganan, M.; Lorentzen, S.B.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sørlie, M. Antibiotic saving effect of combination therapy through synergistic interactions between well-characterized chitooligosaccharides and commercial antifungals against medically relevant yeasts. PLoS ONE 2019, 14, e0227098. [Google Scholar] [CrossRef]
- Peacock, L.; Ferris, V.; Bailey, M.; Gibson, W. Multiple effects of the lectin-inhibitory sugars d-glucosamine and N-acetyl-glucosamine on tsetse-trypanosome interactions. Parasitology 2006, 132, 651–658. [Google Scholar] [CrossRef]
- Ebikeme, C.E.; Peacock, L.; Coustou, V.; Riviere, L.; Bringaud, F.; Gibson, W.C.; Barrett, M.P. N-acetyl-d-glucosamine stimulates growth in procyclic forms of Trypanosoma brucei by inducing a metabolic shift. Parasitology 2008, 135, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.S.J.; Dvorak, J.A. Influence of monosaccharides on the infection of vertebrate cells by Trypanosoma cruzi and Toxoplasma gondii. Mol. Biochem. Parasitol. 1982, 5, 333–341. [Google Scholar] [CrossRef]
- Souto-Padron, T. The surface charge of trypanosomatids. An. Acad. Bras. Cienc. 2002, 74, 649–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, D.; Salomón, C.J.; Lamas, M.C.; Olivieri, A.C. Development of novel formulations for Chagas’ disease: Optimization of benznidazole chitosan microparticles based on artificial neural networks. Int. J. Pharm. 2009, 367, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Nhavene, E.P.F.; da Silva, W.M.; Trivelato Junior, R.R.; Gastelois, P.L.; Venâncio, T.; Nascimento, R.; Batista, R.J.C.; Machado, C.R.; de Macedo, W.A.A.; de Sousa, E.M.B. Chitosan grafted into mesoporous silica nanoparticles as benznidazol carrier for Chagas diseases treatment. Microporous Mesoporous Mater. 2018, 272, 265–275. [Google Scholar] [CrossRef]
- Pujals, G.; Suñé-Negre, J.M.; Pérez, P.; García, E.; Portus, M.; Tico, J.R.; Miñarro, M.; Carrió, J. In vitro evaluation of the effectiveness and cytotoxicity of meglumine antimoniate microspheres produced by spray drying against Leishmania infantum. Parasitol. Res. 2008, 102, 1243–1247. [Google Scholar] [CrossRef]
- Hoseini, M.H.M.; Moradi, M.; Alimohammadian, M.H.; Shahgoli, V.K.; Darabi, H.; Rostami, A. Immunotherapeutic effects of chitin in comparison with chitosan against Leishmania major infection. Parasitol. Int. 2016, 65, 99–104. [Google Scholar] [CrossRef]
- Naderer, T.; Heng, J.; McConville, M.J. Evidence that intracellular stages of Leishmania major utilize amino sugars as a major carbon source. PLoS Pathog. 2010, 6, e1001245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Low molecular weight chitosans-Preparation with the aid of pronase, characterization and their bactericidal activity towards Bacillus cereus and Escherichia coli. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, P.; Yu, W.; Tan, C.; Liu, H.; Xiong, C.; Qiao, Y.; Du, Y. Chitooligosaccharides protect human embryonic hepatocytes against oxidative stress induced by hydrogen peroxide. Mar. Biotechnol. 2010, 12, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Melo-Silveira, R.F.; Fidelis, G.P.; Viana, R.L.S.; Soeiro, V.C.; Da Silva, R.A.; Machado, D.; Costa, L.S.; Ferreira, C.V.; Rocha, H.A.O. Antioxidant and antiproliferative activities of methanolic extract from a neglected agricultural product: Corn cobs. Molecules 2014, 19, 5360–5378. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.; Alder, J.; Al, E. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Approved Standard-M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V.; Ghannoum, M.A.; Espinel-Ingroff, A.; Knapp, C.C.; Ostrosky-Zeichner, L.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; pp. 1–25. [Google Scholar]
- Corral, M.J.; González, E.; Cuquerella, M.; Alunda, J.M. Improvement of 96-well microplate assay for estimation of cell growth and inhibition of Leishmania with Alamar Blue. J. Microbiol. Methods 2013, 94, 111–116. [Google Scholar] [CrossRef]
- Amorim-Carmo, B.; Daniele-Silva, A.; Parente, A.M.S.; Furtado, A.A.; Carvalho, E.; Oliveira, J.W.F.; Santos, E.C.G.; Silva, M.S.; Silva, S.R.B.; Silva-Júnior, A.A.; et al. Potent and Broad-Spectrum Antimicrobial Activity of Analogs from the Scorpion Peptide Stigmurin. Int. J. Mol. Sci. 2019, 20, 623. [Google Scholar] [CrossRef] [Green Version]












| Microorganism | MIC |
|---|---|
| K. pneumoniae (ATCC 10031) | >2 mg/mL |
| P. aeruginosa (ATCC 27853) | 1 mg/mL |
| E. coli (ATCC 25922) | >2 mg/mL |
| S. epidermidis (ATCC 12228) | <0.25 mg/mL |
| E. faecalis (ATCC 29212) | 2 mg/mL |
| S. aureus (ATCC 29213) | 1 mg/mL |
| C. albicans (ATCC 90028) | 0.5 mg/mL |
| C. tropicalis (ATCC 13803) | 1 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.S.d.; Araújo, N.K.; Daniele-Silva, A.; Oliveira, J.W.d.F.; Medeiros, J.M.d.; Araújo, R.M.; Ferreira, L.D.S.; Rocha, H.A.O.; Silva-Junior, A.A.; Silva, M.S.; et al. Antimicrobial Activity of Chitosan Oligosaccharides with Special Attention to Antiparasitic Potential. Mar. Drugs 2021, 19, 110. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020110
Silva NSd, Araújo NK, Daniele-Silva A, Oliveira JWdF, Medeiros JMd, Araújo RM, Ferreira LDS, Rocha HAO, Silva-Junior AA, Silva MS, et al. Antimicrobial Activity of Chitosan Oligosaccharides with Special Attention to Antiparasitic Potential. Marine Drugs. 2021; 19(2):110. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020110
Chicago/Turabian StyleSilva, Nayara Sousa da, Nathália Kelly Araújo, Alessandra Daniele-Silva, Johny Wysllas de Freitas Oliveira, Júlia Maria de Medeiros, Renata Mendonça Araújo, Leandro De Santis Ferreira, Hugo Alexandre Oliveira Rocha, Arnóbio Antônio Silva-Junior, Marcelo Sousa Silva, and et al. 2021. "Antimicrobial Activity of Chitosan Oligosaccharides with Special Attention to Antiparasitic Potential" Marine Drugs 19, no. 2: 110. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020110