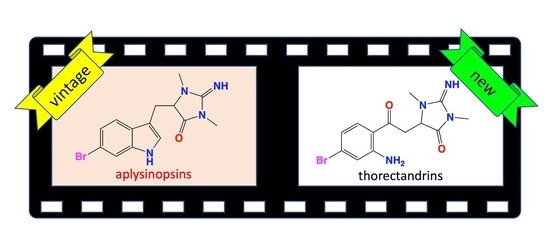
New from Old: Thorectandrin Alkaloids in a Southern Australian Marine Sponge, Thorectandra choanoides (CMB-01889)
Abstract
:1. Introduction
2. Results and Discussion
2.1. GNPS Molecular Networking to Explore New Chemistry
2.2. Thorectandrin A (1)
2.3. Plausible Biosynthetic Pathway
2.4. Other Thorectandrin Co-Metabolites
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Collection and Taxonomy
4.3. Extraction and Fractionation
4.4. Global Natural Product Social (GNPS) Molecular Networking
4.5. Metabolite Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cambie, R.C.; Craw, P.A. Chemistry of Sponges, III. Manoalide Monoacetate and Thorectolide Monoacetate, Two New Sesterterpenoids from Thorectandra excavatus. J. Nat. Prod. 1988, 51, 331–334. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R.J. A New Furanoditerpene from a Southern Australian Marine Sponge, Thorectandra choanoides. Aust. J. Chem. 1995, 48, 1903–1906. [Google Scholar] [CrossRef]
- Charan, R.D.; McKee, T.C.; Boyd, M.R. Thorectandrols A and B, New Cytotoxic Sesterterpenes from the Marine Sponge Thorectandra Species. J. Nat. Prod. 2001, 64, 661–663. [Google Scholar] [CrossRef]
- Charan, R.D.; McKee, T.C.; Boyd, M.R. Thorectandrols C, D, and E, New Sesterterpenes from the Marine Sponge Thorectandra sp. J. Nat. Prod. 2002, 65, 492–495. [Google Scholar] [CrossRef]
- Charan, R.D.; McKee, T.C.; Gustafson, K.R.; Pannell, L.K.; Boyd, M.R. Thorectandramine, a novel β-carboline alkaloid from the marine sponge Thorectandra sp. Tetrahedron Lett. 2002, 43, 5201–5204. [Google Scholar] [CrossRef]
- Charan, R.D.; McKee, T.C.; Boyd, M.R. Cytotoxic Alkaloids from the Marine Sponge Thorectandra sp. Nat. Prod. Res. 2004, 18, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Crews, P. Investigation of Brominated Tryptophan Alkaloids from Two Thorectidae Sponges: Thorectandra and Smenospongia. J. Nat. Prod. 2005, 68, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Khushi, S.; Nahar, L.; Salim, A.A.; Capon, R.J. Trachycladindoles H–M: Molecular Networking Guided Exploration of a Library of Southern Australian Marine Sponges. Aust. J. Chem. 2020, 73, 338. [Google Scholar] [CrossRef]
- Khushi, S.; Nahar, L.; Salim, A.; Capon, R. Cacolides: Sesterterpene Butenolides from a Southern Australian Marine Sponge, Cacospongia sp. Mar. Drugs 2018, 16, 456. [Google Scholar] [CrossRef] [Green Version]
- Khushi, S.; Salim, A.A.; Elbanna, A.H.; Nahar, L.; Bernhardt, P.V.; Capon, R.J. Dysidealactams and Dysidealactones: Sesquiterpene Glycinyl- Lactams, Imides, and Lactones from a Dysidea sp. Marine Sponge Collected in Southern Australia. J. Nat. Prod. 2020, 83, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Zjawiony, J. Aplysinopsins—Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance. Mar. Drugs 2009, 7, 166–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botting, N.P. Chemistry and neurochemistry of the kynurenine pathway of tryptophan metabolism. Chem. Soc. Rev. 1995, 24, 401–412. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Luo, X.; Voogd, N.J.; Li, P.; Li, G. (+)- and (−)-Spiroreticulatine, A Pair of Unusual Spiro Bisheterocyclic Quinoline-imidazole Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata. Org. Lett. 2015, 17, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, X.; Luo, X.; Voogd, N.J.; Li, P.; Li, G. Aplysinopsin-type and Bromotyrosine-derived Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata. Sci. Rep. 2019, 9, 2248. [Google Scholar] [CrossRef] [Green Version]
- Capon, R.J. Extracting value: Mechanistic insights into the formation of natural product artifacts—Case studies in marine natural products. Nat. Prod. Rep. 2020, 37, 55–79. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. Aplysinopsin, a new tryptophan derivative from a sponge. Tetrahedron Lett. 1977, 1, 61–64. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with GNPS. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]










| Position | δC | δH, Mult. (J in Hz) | COSY | HMBC | ROESY |
|---|---|---|---|---|---|
| 3 | 197.8 | ||||
| 3a | 116.1 | ||||
| 4 | 133.9 | 7.67, d (8.7) | 5 | 6, 7a, 3 | 8a, 8b |
| 5 | 119.4 | 6.73, br d (8.7) | 4,7 | 3a, 7 | |
| 6 | 131.1 | ||||
| 7 | 120.7 | 6.97, br s | 5 | 3a, 5, 6 | |
| 7a | 154.1 | ||||
| 8 | 38.0 | a. 3.94, dd (19.0, 3.5) | 8b, 1′ | 1′, 5′, 3 | 4 |
| b. 3.67, dd (19.0, 3.9) | 8a, 1′ | 1′, 3 | 4 | ||
| 1′ | 60.8 | 4.56, dd (3.9, 3.5) | 8a, 8b | 5′, 3 | 2′N-CH3 |
| 2′N-CH3 | 30.3 | 3.09, s | 3′, 1′ | 1′ | |
| 3′ | 160.3 | ||||
| 4′N-CH3 | 26.8 | 3.26, s | 3′, 5′ | ||
| 5′ | 173.8 |
| m/za (M + H) | Molecular Formula | ΔmDa b | MF Difference with 1 |
|---|---|---|---|
| Bromo natural products | |||
| 339 (1) | C13H15BrN4O2 | 0.05 | |
| 367 (1a) | C14H15BrN4O3 | −1.99 | CO |
| 353 (1b) | C14H17BrN4O2 | 0.78 | CH2 |
| Bromo natural product solvolysis adducts | |||
| 397 (1c) | C15H17BrN4O4 | −1.64 | CO + CH2O |
| 355 (1d) | C13H15BrN4O3 | 0.29 | O |
| 369 (1e) | C14H17BrN4O3 | −1.03 | CH2O |
| 383 (1f) | C15H19BrN4O3 | −0.04 | C2H4O |
| 411 (1g) | C17H23BrN4O3 | −0.50 | C4H8O |
| Debromo natural products | |||
| 289 (1h) | C14H16N4O3 | 0.71 | −Br + H + CO |
| 261 (1i) | C13H16N4O2 | −0.57 | −Br + H |
| 303 (1j) | C15H18N4O3 | 1.2 | −Br + H + CH2 |
| Debromo natural product solvolysis adducts | |||
| 277 (1k) | C13H16N4O3 | −1.25 | −Br + H + O |
| 319 (1m) | C15H18N4O4 | 1.1 | −Br + H + CO + OCH2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khushi, S.; Salim, A.A.; Elbanna, A.H.; Nahar, L.; Capon, R.J. New from Old: Thorectandrin Alkaloids in a Southern Australian Marine Sponge, Thorectandra choanoides (CMB-01889). Mar. Drugs 2021, 19, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020097
Khushi S, Salim AA, Elbanna AH, Nahar L, Capon RJ. New from Old: Thorectandrin Alkaloids in a Southern Australian Marine Sponge, Thorectandra choanoides (CMB-01889). Marine Drugs. 2021; 19(2):97. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020097
Chicago/Turabian StyleKhushi, Shamsunnahar, Angela A. Salim, Ahmed H. Elbanna, Laizuman Nahar, and Robert J. Capon. 2021. "New from Old: Thorectandrin Alkaloids in a Southern Australian Marine Sponge, Thorectandra choanoides (CMB-01889)" Marine Drugs 19, no. 2: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/md19020097