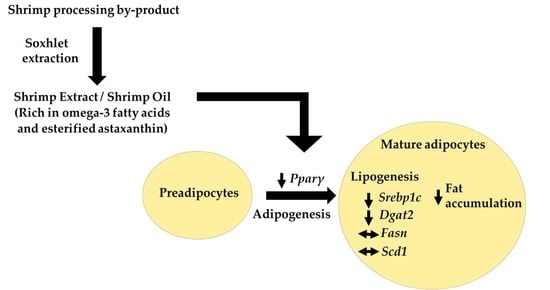
Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results
2.1. Lipid Composition of SO
2.2. Fatty Acids Composition of Oils
2.3. Astaxanthin Content of SO
2.4. SO Reduced Fat Accumulation in 3T3-L1 Adipocytes
2.5. Shrimp Oil Decreased, While Fish Oil Increased the mRNA Expression of Pparγ and Srebp1c in Mature 3T3-L1 Adipocytes
2.6. SO Decreased, While FO Increased the mRNA Expression of Lipogenic Genes and Glut-4 in Mature 3T3-L1 Adipocytes
3. Discussion
4. Materials and Methods
4.1. SO Extraction from Shrimp Processing By-Product
Soxhlet Method
4.2. Lipids and Fatty Acids Composition Analysis of Oils
4.3. Astaxanthin Analysis
4.4. The 3T3-L1 Cell Culture
4.4.1. Materials
4.4.2. Culturing 3T3-L1 Cells
4.4.3. Preparation of Lipid Emulsions to Treat 3T3-L1 Cells
4.4.4. Pilot Study to Establish the Effects of Oil Emulsions on Cell Differentiation
4.4.5. Cell Metabolic Activity
4.4.6. Treatments of 3T3-L1 Preadipocytes with Oil Emulsions to Measure Fat Accumulation, and the Expression of Adipogenic and Lipogenic Genes
4.5. Oil Red O Staining
4.6. Total RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics, Canada, 2015. Adjusting the Scales: Obesity in the Canadian Population after Correcting for Respondent Bias. Available online: https://www150.statcan.gc.ca/n1/pub/82-624-x/2014001/article/11922-eng.htm (accessed on 27 March 2020).
- Statistics, Canada, 2019. Overweight and Obese Adults. 2018. Available online: https://www150.statcan.gc.ca/n1/pub/82-625-x/2019001/article/00005-eng.htm (accessed on 27 March 2020).
- Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Mondal, S.; Seo, H.; Dae Lee, K.; Oh, J. Marine Natural Pigments as Potential Sources for Therapeutic Applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A Systematic Review of Anti-Obesity Medicinal Plants-an Update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhri, S.; Bahrami, G.; Mohammadi, B.; Jalili, R.; Hajialyani, M. Screening and Confirmation of Different Synthetic Adulterants in Slimming Products. Artic. Asian J. Pharm. Clin. Res. 2018, 11, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of Fat Cell Turnover in Humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Iacobellis, G.; Ribaudo, M.C.; Zappaterreno, A.; Iannucci, C.V.; Leonetti, F. Prevalence of Uncomplicated Obesity in an Italian Obese Population. Obes. Res. 2005, 13, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and Adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefterova, M.I.; Lazar, M.A. New Developments in Adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- De Sá, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar] [CrossRef]
- Chong Yew, T. Adipose Tissue Expandability: The Metabolic Problems of Obesity May Arise from the Inability to Become More Obese. Biochem. Soc. Trans. 2008, 36, 935–940. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and Lipid Mobilization in Human Adipose Tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Louveau, I.; Gondret, F. Regulation of Development and Metabolism of Adipose Tissue by Growth Hormone and the Insulin-like Growth Factor System. Domest. Anim. Endocrinol. 2004, 27, 241–255. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S. Cross-Regulation of C/EBPα and PPARγ Controls the Transcriptional Pathway of Adipogenesis and Insulin Sensitivity. Mol. Cell. 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P. SREBP Transcription Factors: Master Regulators of Lipid Homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 Promotes Adipocyte Differentiation and Gene Expression Linked to Fatty Acid Metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sook Sul, H. Transcriptional Regulation of Hepatic Lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, B.; Derraik, J.; Brennan, C. Higher Omega-3 Index Is Associated with Increased Insulin Sensitivity and More Favourable Metabolic Profile in Middle-Aged Overweight Men. Sci. Rep. 2014, 4, 6697. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, Y. Effects of Dietary Polyunsaturated N-3 Fatty Acids on Dyslipidemia and Insulin Resistance in Rodents and Humans. A Review. J. Nutr. Biochem. 2006, 17, 1–13. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K. Health Benefits of Marine Foods and Ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Martinéz, A.; Kiely, M.; Morais, G.; Bandarra, N.M.; Thorsdottir, I. Beneficial Effects of Long-Chain n-3 Fatty Acids Included in an Energy-Restricted Diet on Insulin Resistance in Overweight and Obese European Young Adults. Diabetologia 2008, 51, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Swanson, D.; Block, R. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Subramanian, B.; Thibault, M.H.; Djaoued, Y.; Pelletier, C.; Touaibia, M.; Tchoukanova, N. Chromatographic, NMR and Vibrational Spectroscopic Investigations of Astaxanthin Esters: Application to “Astaxanthin-Rich Shrimp Oil” Obtained from Processing of Nordic Shrimps. Analyst 2015, 140, 7423–7433. [Google Scholar] [CrossRef]
- Vincent, H.; Bourguignon, C.; Weltman, A. Effects of Antioxidant Supplementation on Insulin Sensitivity, Endothelial Adhesion Molecules, and Oxidative Stress in Normal-Weight and Overweight Young. Metabolism 2009, 58, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Sachindra, N.M.; Mahendrakar, N.S. Process Optimization for Extraction of Carotenoids from Shrimp Waste with Vegetable Oils. Bioresour. Technol. 2005, 96, 1195–1200. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Jiao, G.; Hui, J.P.M.; Burton, I.W.; Thibault, M.H.; Pelletier, C.; Boudreau, J.; Tchoukanova, N.; Subramanian, B.; Djaoued, Y.; Ewart, S.; et al. Characterization of Shrimp Oil from Pandalus Borealis by High Performance Liquid Chromatography and High Resolution Mass Spectrometry. Mar. Drugs 2015, 13, 3849–3876. [Google Scholar] [CrossRef] [Green Version]
- De Holanda, H.D.; Netto, F.M. Recovery of Components from Shrimp (Xiphopenaeus Kroyeri) Processing Waste by Enzymatic Hydrolysis. J. Food Sci. 2006, 71, 298–303. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications-A Review. Mar. Drugs. 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus Pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Kim, J.Y.; Jun, H.J.; Kim, S.J.; Lee, J.H.; Hoang, M.H.; Hwang, K.Y.; Um, S.J.; Chang, H.I.; Lee, S.J. The Natural Carotenoid Astaxanthin, a PPAR-α Agonist and PPAR-γ Antagonist, Reduces Hepatic Lipid Accumulation by Rewiring the Transcriptome in Lipid-Loaded Hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Arunkumar, E.; Viswanathan, P.; Anuradha, C.V. Astaxanthin Restricts Weight Gain, Promotes Insulin Sensitivity and Curtails Fatty Liver Disease in Mice Fed a Obesity-Promoting Diet. Process Biochem. 2010, 45, 1406–1414. [Google Scholar] [CrossRef]
- Routray, W.; Dave, D.; Cheema, S.K.; Ramakrishnan, V.V.; Pohling, J. Biorefinery Approach and Environment-Friendly Extraction for Sustainable Production of Astaxanthin from Marine Wastes. Crit. Rev. Biotechnol. 2019, 39, 469–488. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of Lipids and Astaxanthin from Crustacean By-Products: A Review on Supercritical CO2 Extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Vaidya, H.B.; Gangadaran, S.; Cheema, S.K. An Obesogenic Diet Enriched with Blue Mussels Protects against Weight Gain and Lowers Cholesterol Levels in C57BL/6 Mice. Nutr. Res. 2017, 46, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.; Cheema, S.K. Sea Cucumber and Blue Mussel: New Sources of Phospholipid Enriched Omega-3 Fatty Acids with a Potential Role in 3T3-L1 Adipocyte Metabolism. Food Funct. 2014, 5, 3287–3295. [Google Scholar] [CrossRef]
- Shikov, A.; Laakso, I.; Pozharitskaya, O.; Seppänen-Laakso, T.; Krishtopina, A.; Makarova, M.; Vuorela, H.; Makarov, V. Chemical Profiling and Bioactivity of Body Wall Lipids from Strongylocentrotus Droebachiensis. Mar. Drugs 2017, 15, 365. [Google Scholar] [CrossRef] [Green Version]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J.H. Supplementation of Krill Oil with High Phospholipid Content Increases Sum of EPA and DHA in Erythrocytes Compared with Low Phospholipid Krill Oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Yang, C.; Du, S.; Gao, J.-M. A New Method for Determining Free Fatty Acid Content in Edible Oils by Using Electrical Conductivity. Food Anal. Methods 2012, 5, 1453–1458. [Google Scholar] [CrossRef]
- Burgess, E.J.; Perry, N.B.; Killeen, D.P.; Fuller, I.D.; Cumming, A.H.; Card, A.; Barrow, C.J.; Killeen, D.P. Free Fatty Acids in Commercial Krill Oils: Concentrations, Compositions, and Implications for Oxidative Stability. J. Am. Oil Chem. Soc. 2020, 97, 889–900. [Google Scholar] [CrossRef]
- Kim, H.K.; Della-Fera, M.A.; Lin, J.; Baile, C.A. Docosahexaenoic Acid Inhibits Adipocyte Differentiation and Induces Apoptosis in 3T3-L1 Preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manickam, E.; Sinclair, A.J.; Cameron-Smith, D. Suppressive Actions of Eicosapentaenoic Acid on Lipid Droplet Formation in 3T3-L1 Adipocytes. Lipids Health Dis. 2010, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Tanabe, H.; Matsumoto, A.; Takagi, M.; Umegaki, K.; Amagaya, S.; Takahashi, J. Astaxanthin Functions Differently as a Selective Peroxisome Proliferator-Activated Receptor γ Modulator in Adipocytes and Macrophages. Biochem. Pharmacol. 2012, 84, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Matsumoto, C.; Shibano, M.; Fujimori, K. Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes. Nutrients 2018, 10, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα Induces Adipogenesis through PPARγ: A Unified Pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARγ as a Molecular Target of EPA Anti-Inflammatory Activity during TNF-α-Impaired Skeletal Muscle Cell Differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Pérez-Matute, P.; Martínez, J.A.; Marti, A.; Moreno-Aliaga, M.J. Effects of Eicosapentaenoic Acid (EPA) on Adiponectin Gene Expression and Secretion in Primary Cultured Rat Adipocytes. J. Physiol. Biochem. 2006, 62, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of Adipocyte Differentiation and Lipid Accumulation by Stearidonic Acid (SDA) in 3T3-L1 Cells. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, C.L.; Crisma, A.R.; Masi, L.N.; Martins, A.R.; Hirabara, S.M.; Curi, R. DNA Methylation Changes Induced by a High-Fat Diet and Fish Oil Supplementation in the Skeletal Muscle of Mice. J. Nutrigenet. Nutr. 2014, 7, 314–326. [Google Scholar] [CrossRef]
- Vaidya, H.; Cheema, S.K. Arachidonic Acid Has a Dominant Effect to Regulate Lipogenic Genes in 3T3-L1 Adipocytes Compared to Omega-3 Fatty Acids. Food Nutr. Res. 2015, 59, 25866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Takeuchi, Y.; Nakagawa, Y.; Takahashi, H.; Okazaki, H.; Iizuka, Y.; Ohashi, K.; Gotoda, T.; et al. SREBP-1-Independent Regulation of Lipogenic Gene Expression in Adipocytes. J. Lipid Res. 2007, 48, 1581–1591. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Sinclair, A.J.; Cameron-Smith, D.; Barr, D.P.; Molero-Navajas, J.C.; Konstantopoulos, N. Docosapentaenoic Acid (22:5n-3) down-Regulates the Expression of Genes Involved in Fat Synthesis in Liver Cells. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Dentin, R.; Benhamed, F.; Pégorier, J.P.; Foufelle, F.; Viollet, B.; Vaulont, S.; Girard, J.; Postic, C. Polyunsaturated Fatty Acids Suppress Glycolytic and Lipogenic Genes through the Inhibition of ChREBP Nuclear Protein Translocation. J. Clin. Investig. 2005, 115, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.-J. Astaxanthin Reduces Hepatic Lipid Accumulations in High-Fat-Fed C57BL/6J Mice via Activation of Peroxisome Proliferator-Activated Receptor (PPAR) Alpha and Inhibition of PPAR Gamma and Akt. J. Nut. Biochem. 2015, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Voy, B.J.; Urs, S.; Kim, S.; Soltani-Bejnood, M.; Quigley, N.; Heo, Y.R.; Standridge, M.; Andersen, B.; Dhar, M.; et al. The Human Fatty Acid Synthase Gene and De Novo Lipogenesis Are Coordinately Regulated in Human Adipose Tissue. J. Nutr. 2004, 134, 1032–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, C.M.; Ntambi, J.M. Biochemical and Physiological Function of Stearoyl-CoA Desaturase. Am. J. Physiol. Metab. 2009, 297, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntambi, J.M.; Miyazaki, M. Regulation of Stearoyl-CoA Desaturases and Role in Metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Harris, C.A.; Haas, J.T.; Streeper, R.S.; Stone, S.J.; Kumari, M.; Yang, K.; Han, X.; Brownell, N.; Gross, R.W.; Zechner, R.; et al. DGAT Enzymes Are Required for Triacylglycerol Synthesis and Lipid Droplets in Adipocytes. J. Lipid Res. 2011, 52, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Cheol, S.C.; Savage, D.B.; Kulkarni, A.; Xing, X.Y.; Liu, Z.X.; Morino, K.; Kim, S.; Distefano, A.; Samuel, V.T.; Neschen, S.; et al. Suppression of Diacylglycerol Acyltransferase-2 (DGAT2), but Not DGAT1, with Antisense Oligonucleotides Reverses Diet-Induced Hepatic Steatosis and Insulin Resistance. J. Biol. Chem. 2007, 282, 22678–22688. [Google Scholar] [CrossRef] [Green Version]
- Ukropec, J.; Reseland, J.E.; Gasperikova, D.; Demcakova, E.; Madsen, L.; Berge, R.K.; Rustan, A.C.; Klimes, I.; Drevon, C.A.; Sebökova, E. The Hypotriglyceridemic Effect of Dietary N-3 FA Is Associated with Increased β-Oxidation and Reduced Leptin Expression. Lipids 2003, 38, 1023–1029. [Google Scholar] [CrossRef]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-Rich Extract from the Green Alga Haematococcus Pluvialis Lowers Plasma Lipid Concentrations and Enhances Antioxidant Defense in Apolipoprotein E Knockout Mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.C.; Huang, S.C.; Chang, W.T.; Chen, S.C.; Hsu, C.L. Effect of Astaxanthin on the Inhibition of Lipid Accumulation in 3T3-L1 Adipocytes via Modulation of Lipogenesis and Fatty Acid Transport Pathways. Molecules 2020, 25, 3598. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Adipose Tissue and Metabolic Syndrome: Too Much, Too Little or Neither. Eur. J. Clin. Investig. 2015, 45, 1209–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, A.J. The Biochemical Basis of Disease. Essays Biochem. 2018, 62, 619–642. [Google Scholar] [CrossRef] [PubMed]
- Govers, R. Molecular Mechanisms of GLUT4 Regulation in Adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef]
- Peyron-Caso, E.; Fluteau-Nadler, S.; Kabir, M.; Guerre-Millo, M.; Quignard-Boulangé, A.; Slama, G.; Rizkalla, S.W. Regulation of Glucose Transport and Transporter 4 (Glut-4) in Muscle and Adipocytes of Sucrose-Fed Rats: Effects of n-3 Poly- and Monounsaturated Fatty Acids. Horm. Metab. Res. 2002, 34, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane, G.H. A Simple Method For the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Parrish, C.C. Determination of Total Lipid, Lipid Classes, and Fatty Acids in Aquatic Samples. In Lipids in Freshwater Ecosystems; Springer: Berlin, Germany, 1999; pp. 4–20. [Google Scholar] [CrossRef]
- Deibel, D.; Parrish, C.C.; Grønkjær, P.; Munk, P.; Nielsen, T.G. Lipid Class and Fatty Acid Content of the Leptocephalus Larva of Tropical Eels. Lipids 2012, 47, 623–634. [Google Scholar] [CrossRef]
- Chechi, K.; Herzberg, G.R.; Cheema, S.K. Maternal Dietary Fat Intake during Gestation and Lactation Alters Tissue Fatty Acid Composition in the Adult Offspring of C57Bl/6 Mice. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gastélum, J.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Rodríguez-Núñez, J.R.; Correa-Murrieta, M.A.; Sánchez-Duarte, R.G.; Campas-Baypoli, O.N. Astaxanthin and Its Esters in Pigmented Oil from Fermented Shrimp By-Products. J. Aquat. Food Prod. Technol. 2016, 25, 334–343. [Google Scholar] [CrossRef]
- Meyers, S.P.; Bligh, D. Characterization of Astaxanthin Pigments from Heat-Processed Crawfish Waste. J. Agric. Food Chem. 1981, 29, 505–508. [Google Scholar] [CrossRef]
- Vaidya, H.; Goyal, R.K.; Cheema, S.K. Anti-Diabetic Activity of Swertiamarin Is Due to an Active Metabolite, Gentianine, That Upregulates PPAR-γ Gene Expression in 3T3-L1 Cells. Phyther. Res. 2013, 27, 624–627. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Dao, D.T.; Baker, M.A.; Fell, G.L.; Puder, M.; Gura, K.M. Intravenous Fat Emulsion Formulations for the Adult and Pediatric Patient: Understanding the Differences. Nutr. Clin. Pract. 2016, 31, 596–609. [Google Scholar] [CrossRef] [Green Version]
- Meisel, J.A.; Le, H.D.; De Meijer, V.E.; Nose, V.; Gura, K.M.; Mulkern, R.V.; Sharif, M.R.A.; Puder, M. Comparison of 5 Intravenous Lipid Emulsions and Their Effects on Hepatic Steatosis in a Murine Model. J. Pediatr. Surg. 2011, 46, 666–673. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, Z.; Chen, B.; Zhang, B. Oil-in-Water Emulsion Breaking Marine Bacteria for Demulsifying Oily Wastewater. Water Res. 2019, 149, 292–301. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadrich, F.; Sayadi, S. Apigetrin Inhibits Adipogenesis in 3T3-L1 Cells by Downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomczynski, P.; Sacchi, N. The Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction: Twenty-Something Years On. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Akerele, O.A.; Cheema, S.K. A Diet Enriched in Longer Chain Omega-3 Fatty Acids Reduced Placental Inflammatory Cytokines and Improved Fetal Sustainability of C57BL/6 Mice. Prostaglandins Leukot. Essent. Fat. Acids 2018, 137, 43–51. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 C T Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Lipid Composition Wt (%) | Shrimp Oil |
|---|---|
| Wax/Steryl esters | 0.67 ± 0.09 |
| Methyl esters | 0.56 ± 0.06 |
| Ethyl esters | 1.32 ± 0.11 |
| Triacylglycerols | 13.66 ± 5.35 |
| Free fatty acids | 0.33 ± 0.47 |
| Alcohols | 4.46 ± 0.36 |
| Sterols | 21.18 ± 1.69 |
| Phospholipids | 64.20 ± 4.68 |
| Fatty Acids (% nmol/nmol) | Shrimp Oil | Fish Oil |
|---|---|---|
| C14:0 | 0.17 ± 0.00 | 0.09 ± 0.00 |
| C16:0 | 15.73 ± 0.33 | 21.03 ± 0.06 |
| C16:1n7 | 9.58 ± 0.65 | 18.30 ± 0.36 |
| C18:0 | 2.42 ± 0.07 | 2.73 ± 1.13 |
| C18:1n9 | 21.33 ± 1.03 | 5.95 ± 3.51 |
| C18:1n7 | 6.49 ± 1.56 | 8.30 ± 5.78 |
| C18:2n6 | 1.96 ± 0.12 | 1.95 ± 0.04 |
| C18:3n6 | 0.30 ± 0.09 | 0.55 ± 0.01 |
| C18:3n3 | 0.61 ± 0.08 | 1.69 ±0. 03 |
| C20:1n9 | 0.45 ± 0.20 | 0.27 ±0. 00 |
| C20:4n6 | 1.69 ± 0.14 | 1.62 ±0. 03 |
| C20:5n3 | 21.10 ± 0.11 | 20.14 ± 0.4 |
| C22:4n6 | 0.03 ± 0.05 | 0.33 ±0. 00 |
| C22:5n3 | 1.48 ± 0.11 | 3.28 ±0. 07 |
| C22:6n3 | 13.89 ± 0.13 | 13.77 ± 0.28 |
| ∑SFA | 18.34 ± 0.25 | 23.85 ± 1.06 |
| ∑MUFA | 40.58 ± 0.09 | 32.82 ± 1.89 |
| ∑n-3 PUFA | 37.09 ± 0.04 | 38.88 ± 0.8 |
| ∑n-6 PUFA | 3.99 ± 0.11 | 4.44 ± 0.03 |
| Fraction | Concentration (μg/mL Shrimp Extract) | Astaxanthin Yield (μg/g Shrimp Processing By-Product) |
|---|---|---|
| Free Astx | 8.24 | 24.03 |
| Astx-E | 64.37 | 187.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phadtare, I.; Vaidya, H.; Hawboldt, K.; Cheema, S.K. Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes. Mar. Drugs 2021, 19, 259. https://0-doi-org.brum.beds.ac.uk/10.3390/md19050259
Phadtare I, Vaidya H, Hawboldt K, Cheema SK. Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes. Marine Drugs. 2021; 19(5):259. https://0-doi-org.brum.beds.ac.uk/10.3390/md19050259
Chicago/Turabian StylePhadtare, Indrayani, Hitesh Vaidya, Kelly Hawboldt, and Sukhinder Kaur Cheema. 2021. "Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes" Marine Drugs 19, no. 5: 259. https://0-doi-org.brum.beds.ac.uk/10.3390/md19050259