Seaweeds and Their Natural Products for Preventing Cardiovascular Associated Dysfunction
Abstract
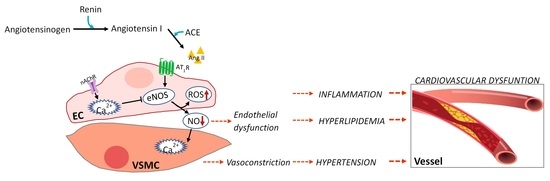
:1. Introduction
2. Marine Natural Product on Hyperlipidemia
3. Marine Natural Products Affect Endothelial Dysfunction
4. Marine Natural Products Inhibit ACE
5. Marine Natural Products Affect ACE2 for SARS-CoV-2 Entry into Cells
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Wong, N.D.; Lopez, V.; Tang, S.; Williams, G.R. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am. J. Cardiol. 2006, 98, 204–208. [Google Scholar] [CrossRef]
- Zou, Z.; Cini, K.; Dong, B.; Ma, Y.; Ma, J.; Burgner, D.P.; Patton, G.C. Time trends in cardiovascular disease mortality across the BRICS: An age-period-cohort analysis of key nations with emerging economies using the global burden of disease study 2017. Circulation 2020, 141, 790–799. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, J.F.; Wadhera, R.K.; Lee, D.; Yeh, R.W.; Sommers, B.D. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts: Study examines community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff. 2020, 39, 1984–1992. [Google Scholar] [CrossRef]
- Rate, C.-F.; Onder, G.; Rezza, G.; Brusaferro, S. Characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020, 23, 1775–1776. [Google Scholar]
- Bonow, R.O.; O’Gara, P.T.; Yancy, C.W. Cardiology and COVID-19. JAMA 2020, 324, 1131–1132. [Google Scholar] [CrossRef]
- Colling, M.E.; Kanthi, Y. COVID–19-associated coagulopathy: An exploration of mechanisms. Vasc. Med. 2020, 25, 471–478. [Google Scholar] [CrossRef]
- Moran, A.E.; Roth, G.A.; Narula, J.; Mensah, G.A. 1990–2010 global cardiovascular disease atlas. Glob. Heart 2014, 9, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Lopez, E.O.; Ballard, B.D.; Jan, A. Cardiovascular Disease. StatPearls, 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK535419/ (accessed on 5 September 2021).
- Eaton, C.B.; Feldman, H.A.; Assaf, A.R.; McPhillips, J.B.; Hume, A.L.; Lasater, T.M.; Levinson, P.; Carleton, R.A. Prevalence of hypertension, dyslipidemia, and dyslipidemic hypertension. J. Fam. Pract. 1994, 38, 17–24. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003, 289, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Prevention of cardiovascular disease through modulation of endothelial cell function by dietary seaweed intake. Phytomed. Plus 2021, 2, 100026. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. 2020, 11, 6777–6806. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Sabirin, F.; Soo, K.K.; Ziau, H.S.; Kuen, L.S. Antihypertensive effects of edible brown seaweeds in rats. Int. J. Adv. Appl. Sci. 2016, 3, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Cho, Y.-R.; Park, G.-M.; Won, K.-B.; Ann, S.H.; Yang, D.H.; Kang, J.-W.; Lim, T.-H.; Kim, H.-K.; Choe, J. High-density lipoprotein cholesterol and the risk of obstructive coronary artery disease beyond low-density lipoprotein cholesterol in non-diabetic individuals. Eur. J. Prevent. Cardiol. 2020, 27, 706–714. [Google Scholar] [CrossRef]
- Navar-Boggan, A.M.; Peterson, E.D.; D’Agostino Sr, R.B.; Neely, B.; Sniderman, A.D.; Pencina, M.J. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015, 131, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Jin, Q.; Yu, H.; Li, P. The evaluation and utilization of marine-derived bioactive compounds with anti-obesity effect. Curr. Med. Chem. 2018, 25, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.-J.; Morcillo, M.; Tenorio, M.-D.; Mateos-Aparicio, I.; Andrés, V.; Redondo-Cuenca, A. Health-promoting effects in the gut and influence on lipid metabolism of Himanthalia elongata and Gigartina pistillata in hypercholesterolaemic Wistar rats. Eur. Food Res. Technol. 2014, 238, 409–416. [Google Scholar] [CrossRef]
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs 2015, 13, 788–805. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant and hypolipidaemic properties of red seaweed, Gracilaria changii. J. Appl. Phycol. 2014, 26, 987–997. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K. Insulinotrophic and hypolipidemic effects of Ecklonia cava in streptozotocin-induced diabetic mice. Asian Pac. J. Trop. Med. 2012, 5, 374–379. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Ruqqia, K.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S.; Athar, M. Hypolipidaemic potential of seaweeds in normal, triton-induced and high-fat diet-induced hyperlipidaemic rats. J. Appl. Phycol. 2015, 27, 571–579. [Google Scholar] [CrossRef]
- Cuong, H.D.; Thuy, T.T.; Huong, T.T.; Ly, B.M.; Van, T.T. Structure and hypolipidaemic activity of fucoidan extracted from brown seaweed Sargassum henslowianum. Nat. Prod. Res. 2015, 29, 411–415. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Bogdanovich, L.N.; Ivanova, T.B.; Byankina, A.O.; Kryzhanovskiy, S.P.; Yermak, I.M. Effect of carrageenan food supplement on patients with cardiovascular disease results in normalization of lipid profile and moderate modulation of immunity system markers. PharmaNutrition 2014, 2, 33–37. [Google Scholar] [CrossRef]
- Dousip, A.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H.; Lim, T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2014, 26, 999–1008. [Google Scholar] [CrossRef]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Matloub, A.; Aly, H.; El, A.; Farrag, R.; Fouad, G.I. Hypolipidemic and anti-atherogenic effect of sulphated polysaccharides from the green alga Ulva fasciata. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 1–12. [Google Scholar]
- Hassan, S.; El-Twab, S.A.; Hetta, M.; Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, M.H.; Kim, J.-Y.; Lee, J.H.; You, S.; Lee, S.-J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205. [Google Scholar] [CrossRef]
- Park, J.; Yeom, M.; Hahm, D.-H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Woo, M.-N.; Jeon, S.-M.; Kim, H.-J.; Lee, M.-K.; Shin, S.-K.; Shin, Y.C.; Park, Y.-B.; Choi, M.-S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem.-Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal polysaccharides as therapeutic agents for atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [Green Version]
- Bhakuni, D.S.; Silva, M. Biodynamic substances from marine flora. Bot. Mar. 1974, 17, 40–51. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Hossain, M.M.; Rahman, M.M.; Subhan, N.; Mamun, M.A.A.; Ulla, A.; Reza, H.M.; Alam, M.A. Astaxanthin prevented oxidative stress in heart and kidneys of isoproterenol-administered aged rats. J. Diet. Suppl. 2018, 15, 42–54. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Cai, W.; Lin, Y.-L.; Lin, Q.-F.; Jiang, Q.; Lin, Z.; Chen, L.-L. Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats. Arzneimittelforschung 2011, 61, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Han, J.-S.; Heo, S.-J.; Hwang, J.-Y.; Jeon, Y.-J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, T.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of eckol and its derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3710–3712. [Google Scholar]
- Lu, Y.A.; Jiang, Y.; Yang, H.W.; Hwang, J.; Jeon, Y.J.; Ryu, B. Diphlorethohydroxycarmalol Isolated from Ishige okamurae Exerts Vasodilatory Effects via Calcium Signaling and PI3K/Akt/eNOS Pathway. Int. J. Mol. Sci. 2021, 22, 1610. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, V.S.; Kurup, G.M. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica protects heart by ameliorating hyperlipidemia, endothelial dysfunction and inflammation in isoproterenol induced experimental myocardial infarction. J. Funct. Foods 2019, 54, 22–31. [Google Scholar] [CrossRef]
- Zaman, M.A.; Oparil, S.; Calhoun, D.A. Drugs targeting the renin–angiotensin–aldosterone system. Nat. Rev. Drug Discov. 2002, 1, 621–636. [Google Scholar] [CrossRef]
- Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Screening of extracts from red algae in Jeju for potentials MarineAngiotensin-I converting enzyme (ACE) inhibitory activity. Algae 2006, 21, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.; Ko, S.-C.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, M.C.; Kang, N.; Kim, H.-S.; Lee, S.-H.; Ahn, G.; Jung, W.-K.; Jeon, Y.-J. Effect of angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO) production of 6,6′-bieckol, a marine algal polyphenol and its anti-hypertensive effect in spontaneously hypertensive rats. Process Biochem. 2017, 58, 326–332. [Google Scholar] [CrossRef]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.W. Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: Low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Res. Int. 2017, 99 Pt 2, 950–958. [Google Scholar] [CrossRef]
- Olivares-Molina, A.; Fernández, K. Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J. Appl. Phycol. 2016, 28, 1295–1302. [Google Scholar] [CrossRef]
- Zhu, H.B.; Geng, M.Y.; Guan, H.S.; Zhang, J.T. Antihypertensive effects of D-polymannuronic sulfate and its related mechanisms in renovascular hypertensive rats. Acta Pharmacol. Sin. 2000, 21, 727–732. [Google Scholar] [PubMed]
- Makkar, F.; Chakraborty, K. Antioxidative sulphated polygalactans from marine macroalgae as angiotensin-I converting enzyme inhibitors. Nat. Prod. Res. 2018, 32, 2100–2106. [Google Scholar] [CrossRef]
- Mourad, J.-J.; Levy, B.I. Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat. Rev. Cardiol. 2020, 17, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syahputra, G.; Gustini, N.; Bustanussalam, B.; Hapsari, Y.; Sari, M.; Ardiansyah, A.; Bayu, A.; Putra, M.Y. Molecular docking of secondary metabolites from Indonesian marine and terrestrial organisms targeting SARS-CoV-2 ACE-2, M pro, and PL pro receptors. Pharmacia 2021, 68, 533–560. [Google Scholar] [CrossRef]
- Salih, A.E.M.; Thissera, B.; Yaseen, M.; Hassane, A.S.I.; El-Seedi, H.R.; Sayed, A.M.; Rateb, M.E. Marine sulfated polysaccharides as promising antiviral agents: A comprehensive report and modeling study focusing on SARS CoV-2. Mar. Drugs 2021, 19, 406. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive alkaloids from Genus Aspergillus: Mechanistic interpretation of their antimicrobial and potential SARS-CoV-2 inhibitory activity using molecular modelling. Int. J. Mol. Sci. 2021, 22, 1866. [Google Scholar] [CrossRef] [PubMed]


| Seaweeds | Experimental Models | Effects (% or mmol/L) | Ref. |
|---|---|---|---|
| Himanthalia elongate, B | Hypercholesterolaemic wistar rats : 21% in diets for four weeks | ↓: TG by 28% ↑: HDL-C by 20% | [24] |
| Gigartina pistillata, R | Hypercholesterolaemic wistar rats : 23% in diets for four weeks | ↓: TG by 30%, TC by 18%, LDL-C by 16% | [24] |
| Derbesia tenuissima, G | High-Fat Fed Rats : 5% in diets for eight weeks | ↓: TG by 38% and TC by 17% | [25] |
| Gracilaria changii, R | High-cholesterol/high-fat Sprague Dawley rats : 5% or 10% in diets for eight weeks | 5% ↓: TC by 39.19%, LDL-C by 36.36%, TG by 25.45% 10% ↓: TC by 40.34%, LDL-C by 35.95%, TG by 30.91% | [26] |
| Ecklonia cava, B | STZ-diabetic mice : 5% in diets for four weeks | ↓: TG by 72%, TC by 53%, and LDL-C by 78% | [27] |
| Ecklonia stolonifera, B | 3T3-L1 preadipocyte cells : Phloroglucinol, Eckol, Dieckol, Dioxinodehydroeckol, Phlorofucofuroeckol A, 12.5 to 100 µM, eight days | ↓: lipid accumulation. ↓: level of adipocyte marker genes | [28] |
| Rhizoclonium implexum, G | A: Adult Albino rats (Sprauge-Dawley) T: Triton-induced hyperlipidaemic rats H: High-fat diet-induced hyperlipidaemic rats : 10 mg/200 g/day for 12 days, OA | A: ↓: TC by 14.4%, TG by 26.4%, LDL-C by 25.5% ↑: HDL-C by 3.1% | [29] |
| Dictyota Indica, B | A:↓: TC by 13.5%, TG by 24.6%, LDL-C by 25.4% ↑: HDL-C by 3.1% | ||
| Padina pavonia, B | A: ↓: TC by 26.5%, TG by 37%, LDL-C by 54.3% ↑: HDL-C by 23.5% | ||
| Stoechospermum marginatum, B | A: ↓: TC by 21.7%, TG by 40.2%, LDL-C by 30% ↑: HDL-C by 6.2% | ||
| Stokeyia indica, B | A: ↓: TC by 22.6%, TG by 17.2%, LDL-C by 40.9% ↑: HDL-C by 0.7% | ||
| Jolyna laminarioides, B | A: ↓: TC by 10%, TG by 49%, LDL-C by 28.7% ↑: HDL-C by 23.5% | ||
| T: ↓: TC by 41.2%, TG by 25.2%, LDL-C by 92.4% ↑: HDL-C by 60.6% | |||
| H: ↓: TC by 19.8%, TG by 31.6%, LDL-C by 34.5% ↑: HDL-C by 33.1% | |||
| Sargassum binderi, B | A: ↓: TC by 20.5%, TG by 4.2%, LDL-C by 28.0%, HDL-C by 17.4% | ||
| T: ↓: TC by 37.6%, TG by 52.2%, LDL-C by 51.1% ↑: HDL-C by 8.6% | |||
| H: ↓: TC by 2.5%, TG by 33%, LDL-C by 2.9% ↑: HDL-C by 30% | |||
| Melanothamnus afaqhusainii, R | A: ↓: TC by 10.3%, TG by 36.1%, LDL-C by 17.5% ↑: HDL-C by 5% | ||
| T: ↓: TC by 35.2%, TG by 43.2%, LDL-C by 71.4% ↑: HDL-C by 57.3% | |||
| H: ↓: TC by 14.2%, TG by 25.1%, LDL-C by 5.4% ↑: HDL-C by 16.8% | |||
| Fucoidan from Sargassum henslowianum (B) | High-fat diet albino mice of BALB/c strain : 100 mg/kg/day for four weeks, OA | ↓: TC by 21.09%, TG by 6.35%, LDL-C by 18.74% | [30] |
| Carrageenans | Ischemic Heart Disease (IHD) patients : 250 mg/day for 20 days, OA | ↓: TC by 16.5%, LDL-C by 33.5% | [31] |
| Kappaphycus alvarezii, R | High-cholesterol diet Male Sprague–Dawley rats : 300 mg/kg/day for eight weeks, OA | ↓: TC by 1.91±0.62%, TG by 0.65±0.05, LDL-C by 1.65±0.08 (mmol/L) ↑: HDL-C by 1.74±0.08 (mmol/L) | [32] |
| Sargassum polycystum, B | ↓: TC by 1.91±0.62%, TG by 0.65±0.05, LDL-C by 1.65±0.08 (mmol/L) ↑: HDL-C by 1.74±0.08 (mmol/L) | ||
| Ulva fasciata, G | High-cholesterol diet rats : 175 mg/kg/day for four weeks, OA | ↓: TC by 46.43%, TG by 69.03%, LDL-C by 81.04% ↑: HDL-C by 668.31% | [33] |
| Ulva lactuca, G | Hypercholesterolemic diet rats : 250 mg/kg/day for four weeks, OA | ↑: HDL-C by 180% | [34] |
| Monostroma nitidum, G | lipid-loaded hepatocytes (HepG2 cell line) : 200 µg/mL for one day | ↓: Cellular cholesterol by 36%, TG by 31%, | [35] |
| Fucoidan | Hyperlipidemic diet mice : 10 to 50 mg/kg/day for four weeks, OA | ↓: TC, TG and LDL-C ↑: HDL-C | [36] |
| Fucoxanthin | Hyperlipidemic diet mice : 21% in diets for six weeks, OA | ↓: Liver TG synthesis, adipocyte fatty acid synthesis, and cholesterol-regulating enzyme activity ↑: Plasma HDL-C ↑: Fecal TG level | [37] |
| Component | Experimental Model | Effects | Ref |
|---|---|---|---|
| Astaxanthin | ISO-induced myocardial infarction and cardiac hypertrophy model in rats : 25 mg/kg/day for two weeks, OA | ↓: ROS generation in heart tissue ↓: Oxidative damage ↑: Antioxidant enzyme activity | [42] |
| STZ-induced diabetes in male rats : 10 mg/kg/d, OA | ↓: Blunted endothelium-dependent vasodilator responses to Ach ↓: Aorta-induced oxidative stress and LOX-1 levels ↑: eNOS levels | [43] | |
| Dieckol | High glucose stimulation in cultured vascular endothelial cells. : 10 or 50 μg/mL | ↓: ROS production ↓: iNOS, COX-2, and NF-κB levels | [44] |
| Eckol and its derivates | Cultured vascular endothelial cells/mice : 50∼200 μg/mL | Protects the vascular barrier | [45] |
| DPHC from Ishige okamurae | Cultured vascular endothelial (EA.hy926) cells)/Tg(flk:EGFP) Transgenic Zebrafish : 100 μM/0.6 μM | ↑: Ach receptor and VEGF receptor 2 ↑: NO production ↑: Ca2+ release ↑: Endothelium vasodilation | [46] |
| Sulfated polysaccharides from Padina tetrastromatica | ISO induced myocardial infarction in rats : 50 mg/kg/day for 12 days, OA | ↓: hyperlipidemia ↓: Endothelial dysfunction ↓: Inflammatory reactions | [47] |
| Seaweed Species | Extraction/Compound | Inhibition | Ref |
|---|---|---|---|
| Twenty-six red algae | Aqueous extract at 20 °C | IC50 (μg/mL) = Lomentaria catenate, 13.78; Lithophyllum okamurae, 12.21; (* IC50 of captopril is 0.05 ± 0.8 μg/mL) | [49] |
| Ecklonia sp, B | Ethanol extract of Ecklonia cava, Phloroglucinol, Triphlorethol-A, Phlorofucofuroeckol A, Dieckol, Eckol, Eckstolonol, and 6,6′-bieckol | IC50 = Ecklonia cava EtOH extract, 0.96 mg/mL Phloroglucinol, 2.57 ± 0.09 Triphlorethol-A, 2.01 ± 0.36 Phlorofucofuroeckol A, 12.74 ± 0.15 μM Dieckol, 1.47 ± 0.04 Eckol, 2.01 ± 0.36 Eckstolonol, 2.95 ± 0.28 6,6′-bieckol, 0.42 mM | [50,51,52] |
| Sargassum wightii, B | Ethyl acetate extract (Polyphenol-rich extract (49.10 ± 2.52 µg/mg) | IC50 = 56.96 µg/mL | [53] |
| Sargassum siliquosum, B | MeOH extract (Fucoxanthin-rich fractions) | IC50 = 0.94 ± 0.67 mg/mL | [54] |
| Lessonia nigrescens, B | Maceration | IC50 = 48.57 ± 8.04 μg/mg | [55] |
| Cellulase | IC50 = 19.71 ± 2.04 μg/mg | ||
| α-Amylase | IC50 = 10.10 ± 1.55 μg/mg | ||
| Macrocystis pyrifera, B | Cellulase | IC50 = 82.87 ± 8.82 μg/mg | |
| α-Amylase | IC50 = 11.93 ± 0.94 μg/mg | ||
| Durvillaea antarctica, B | α-Amylase | IC50 = 7.80 ± 0.69 μg/mg | |
| Pelvetia canaliculata, B | D-Polymannuronic sulphate | ↑ production of NO; ↓ concentrations of Ang II and ET 1; ↓ Blood pressure; | [56] |
| Kappaphycus alvarezii, R | Sulphated polygalactans | IC50 = 0.02 μg/mL | [57] |
| Gracilaria opuntia, R | Sulphated polygalactans | IC50 = 0.70 μg/mL |
| Binding Free Energy Value (∆G) for hACE-2 | Chemical Structure | Ref | |
|---|---|---|---|
| Dieckol (Ecklonia cava) | −10.23 a |  | [59] |
| Phlorofucofuroeckol A (Ecklonia sp.) | −9.73 a |  | |
| Fucoidan (Brown algae) | −4.9 a |  | |
| Thalassodendrone (Thalassodendrin ciliatum) | −8.65 a |  | |
| Thalassiolin D (Thalassodendrin sp.) | −8.21 a |  | |
| Sulfated polymannuroguluronate (SPMG) | −5.4 b |  | [60] |
| lambda-carrageenan | −5.0 b |  | |
| Heparin | −5.0 b |  | |
| Fumitremorgin C C | −2.86 |  | [61] |
| 12,13-Dihydroxy fumitremorgin C C | −2.88 |  | |
| Fumigatoside E C | −21.17 |  | |
| Fumigatoside F C | −13.81 |  | |
| Versicoloid A C | −1.86 |  | |
| Versicoloid B C | −2.66 | ||
| Aspergicin C | −17.66 |  | |
| Stephacidin A C | −0.584 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.; Kim, Y.-S.; Jeon, Y.-J. Seaweeds and Their Natural Products for Preventing Cardiovascular Associated Dysfunction. Mar. Drugs 2021, 19, 507. https://0-doi-org.brum.beds.ac.uk/10.3390/md19090507
Ryu B, Kim Y-S, Jeon Y-J. Seaweeds and Their Natural Products for Preventing Cardiovascular Associated Dysfunction. Marine Drugs. 2021; 19(9):507. https://0-doi-org.brum.beds.ac.uk/10.3390/md19090507
Chicago/Turabian StyleRyu, Bomi, Young-Sang Kim, and You-Jin Jeon. 2021. "Seaweeds and Their Natural Products for Preventing Cardiovascular Associated Dysfunction" Marine Drugs 19, no. 9: 507. https://0-doi-org.brum.beds.ac.uk/10.3390/md19090507