Pigment Composition of Nine Brown Algae from the Iberian Northwestern Coastline: Influence of the Extraction Solvent
Abstract
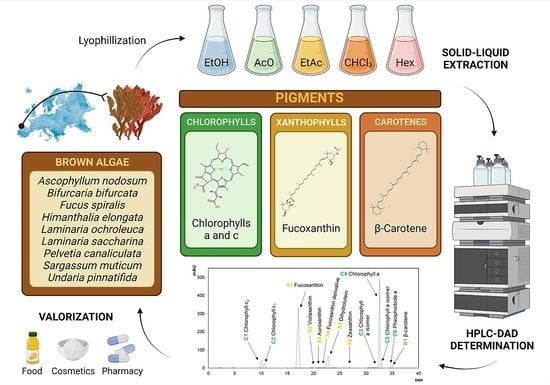
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Sample Collection
3.2. Sample Extraction
3.3. Chromatographic Analysis of Algal Pigments via HPLC-DAD
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of bioactive properties in brown algae from the northwest iberian peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Losada-Lopez, C.; Dopico, D.C.; Faína-Medín, J.A. Neophobia and seaweed consumption: Effects on consumer attitude and willingness to consume seaweed. Int. J. Gastron. Food Sci. 2021, 24, 100338. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Cardoso, L.C.; Ghiggi, V.; Woiciechowski, A.L.; De Souza Vandenberghe, L.P.; Soccol, C.R. Microbial Pigments. In Biotransformation of Waste Biomass into High Value Biochemicals; Springer: New York, NY, USA, 2014; Volume 9781461480, pp. 73–97. ISBN 9781461480051. [Google Scholar]
- Kuda, T.; Nishizawa, M.; Toshima, D.; Matsushima, K.; Yoshida, S.; Takahashi, H.; Kimura, B.; Yamagishi, T. Antioxidant and anti-norovirus properties of aqueous acetic acid macromolecular extracts of edible brown macroalgae. LWT 2021, 141, 110942. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Nitschke, U.; Stengel, D.B. Acclimation potential and biochemical response of four temperate macroalgae to light and future seasonal temperature scenarios. Algal Res. 2021, 54, 102190. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The use of invasive algae species as a source of secondary metabolites and biological activities: Spain as case-study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Anti-glycation effects of brown algae extracts and its phenolic compounds. Food Biosci. 2021, 41, 101042. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown algae carbohydrates: Structures, pharmaceutical properties, and research challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine sulfated polysaccharides: Preventive and therapeutic effects on metabolic syndrome. Mar. Drugs 2021, 19, 608. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: https://www.govinfo.gov/app/details/CFR-2012-title21-vol3/CFR-2012-title21-vol3-sec184-1120/summary (accessed on 13 December 2021).
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown algae potential as a functional food against hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef]
- Smith, J.L.; Summers, G.; Wong, R. Nutrient and heavy metal content of edible seaweeds in New Zealand. N. Z. J. Crop Hortic. Sci. 2010, 38, 19–28. [Google Scholar] [CrossRef]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef]
- Büchel, C. Light harvesting complexes in chlorophyll c-containing algae. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148027. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Pandey, A.K. Analysis of Chlorophylls; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164556. [Google Scholar]
- Zeb, A.; Haq, A.; Murkovic, M. Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (chicory) leaves. Eur. Food Res. Technol. 2019, 245, 365–374. [Google Scholar] [CrossRef]
- Činčárová, D.; Hájek, J.; Dobřichovský, M.; Lukeš, M.; Hrouzek, P. Recommendations on the quantitative analysis of pheophorbides, photosensitizers present in algal biomass intended as food supplement. Algal Res. 2021, 56, 102298. [Google Scholar] [CrossRef]
- Erdoğan, A.; Karataş, A.B.; Demirel, Z.; Dalay, M.C. Purification of fucoxanthin from the diatom Amphora capitellata by preparative chromatography after its enhanced productivity via oxidative stress. J. Appl. Phycol. 2021, 1–9. [Google Scholar] [CrossRef]
- Steingass, C.B.; Vollmer, K.; Lux, P.E.; Dell, C.; Carle, R.; Schweiggert, R.M. HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Res. Int. 2020, 127, 108709. [Google Scholar] [CrossRef]
- Dumont, D.; Danielato, G.; Chastellier, A.; Saint Oyant, L.H.; Fanciullino, A.L.; Lugan, R. Multi-targeted metabolic profiling of carotenoids, phenolic compounds and primary metabolites in goji (Lycium spp.) berry and tomato (Solanum lycopersicum) reveals inter and intra genus biomarkers. Metabolites 2020, 10, 422. [Google Scholar] [CrossRef]
- Gallego, R.; Tardif, C.; Parreira, C.; Guerra, T.; Alves, M.J.; Ibáñez, E.; Herrero, M. Simultaneous extraction and purification of fucoxanthin from Tisochrysis lutea microalgae using compressed fluids. J. Sep. Sci. 2020, 43, 1967–1977. [Google Scholar] [CrossRef]
- Latasa, M.; Scharek, R.; Le Gall, F.; Guillou, L. Pigment suites and taxonomic groups in Prasinophyceae. J. Phycol. 2004, 40, 1149–1155. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. X 2020, 6, 100092. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (Chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhan, C.H.; Maoka, T.; Wada, Y.; Koyama, Y. Fabrication of dye-sensitized solar cells using chlorophylls c1 and c2 and their oxidized forms c1′ and c2′ from Undaria pinnatifida (Wakame). Chem. Phys. Lett. 2007, 447, 79–85. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Stengel, D.B. Intra-thallus differentiation of fatty acid and pigment profiles in some temperate Fucales and Laminariales. J. Phycol. 2015, 51, 25–36. [Google Scholar] [CrossRef]
- Lewey, S.A.; Gorham, J. Pigment composition and photosynthesis in Sargassum muticum. Mar. Biol. 1984, 80, 109–115. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Ecological and commercial implications of temporal and spatial variability in the composition of pigments and fatty acids in five Irish macroalgae. Mar. Biol. 2017, 164, 158. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hanelt, D.; Kräbs, G.; Wiencke, C. Morphology, growth, photosynthesis and pigments in Laminaria ochroleuca (Laminariales, Phaeophyta) under ultraviolet radiation. Phycologia 2004, 43, 603–613. [Google Scholar] [CrossRef]
- Rajauria, G. In-vitro antioxidant properties of lipophilic antioxidant compounds from 3 brown seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Sun, X.; Sun, X.; Wang, S.; Xu, Y. Fucoxanthin Isolated from Undaria pinnatifida can interact with Escherichia coli and Lactobacilli in the intestine and inhibit the growth of pathogenic bacteria. J. Ocean Univ. China 2019, 18, 926–932. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant content and activity of the seaweed Saccharina latissima: A seasonal perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, A.; Tørring, D.; Thomsen, M.; Canal-Vergés, P.; Nielsen, M.; Rasmussen, M.; Eybye, K.; Larsen, M.; Balsby, T.; Petersen, J. Impact of environmental conditions on biomass yield, quality, and bio-mitigation capacity of Saccharina latissima. Aquac. Environ. Interact. 2016, 8, 619–636. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, J.; de Quirós, A.R.B. Evaluation of Bioactive Compounds in Seaweeds: A Review. In Seaweeds: Agricultural Uses, Biological and Antioxidant Agents; Springer: Berlin/Heidelberg, Germany, 2014; Volume 5, pp. 99–113. ISBN 9781631175756. [Google Scholar]
- Amorim-Carrilho, K.; Lage-Yusty, M.A.; López-Hernández, J. Variation of bioactive compounds in dried seaweed Himanthalia elongata subjected to different culinary processes. CYTA J. Food 2014, 12, 336–339. [Google Scholar] [CrossRef]
- de Quirós, A.R.B.; Frecha-Ferreiro, S.; Vidal-Pérez, A.M.; López-Hernández, J. Antioxidant compounds in edible brown seaweeds. Eur. Food Res. Technol. 2010, 231, 495–498. [Google Scholar] [CrossRef]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Pigments and Minor Compounds in Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 205–251. ISBN 9780857095121. [Google Scholar]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2017, 29, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Vega, J.; Álvarez-Gómez, F.; Güenaga, L.; Figueroa, F.L.; Gómez-Pinchetti, J.L. Antioxidant activity of extracts from marine macroalgae, wild-collected and cultivated, in an integrated multi-trophic aquaculture system. Aquaculture 2020, 522, 735088. [Google Scholar] [CrossRef]
- Collén, J.; Davison, I.R. Reactive oxygen metabolism in intertidal Fucus spp. (Phaeophyceae). J. Phycol. 1999, 35, 62–69. [Google Scholar] [CrossRef]
- Rosa, G.P.; Barreto, M.C.; Seca, A.M.L. Pharmacological effects of Fucus spiralis extracts and phycochemicals: A comprehensive review. Bot. Mar. 2019, 62, 167–178. [Google Scholar] [CrossRef]
- Andersen, G.S.; Pedersen, M.F.; Nielsen, S.L. Temperature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). J. Phycol. 2013, 49, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.M.; Manns, D.; D’Este, M.; Krause-Jensen, D.; Rasmussen, M.B.; Larsen, M.M.; Alvarado-Morales, M.; Angelidaki, I.; Bruhn, A. Variation in biochemical composition of Saccharina latissima and Laminaria digitata along an estuarine salinity gradient in inner Danish waters. Algal Res. 2016, 13, 235–245. [Google Scholar] [CrossRef]
- Boderskov, T.; Schmedes, P.S.; Bruhn, A.; Rasmussen, M.B.; Nielsen, M.M.; Pedersen, M.F. The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. J. Appl. Phycol. 2016, 28, 1153–1165. [Google Scholar] [CrossRef]
- Vilg, J.V.; Nylund, G.M.; Werner, T.; Qvirist, L.; Mayers, J.J.; Pavia, H.; Undeland, I.; Albers, E. Seasonal and spatial variation in biochemical composition of Saccharina latissima during a potential harvesting season for Western Sweden. Bot. Mar. 2015, 58, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Sousa, G.; Trifunovska, M.; Antunes, M.; Miranda, I.; Moldão, M.; Alves, V.; Vidrih, R.; Lopes, P.A.; Aparicio, L.; Neves, M.; et al. Optimization of ultrasound-assisted extraction of bioactive compounds from Pelvetia canaliculata to sunflower oil. Foods 2021, 10, 1732. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Soares, C.; Figueiredo, I.; Sousa, B.; Torres, A.C.; Sousa-Pinto, I.; Veiga, P.; Rubal, M.; Fidalgo, F. Fucoid macroalgae have distinct physiological mechanisms to face emersion and submersion periods in their southern limit of distribution. Plants 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Y.; Yang, S.; Wang, Y.; Cui, Q.M. Effect of Temperature on the Growth and Biochemical Composition of Sargassum muticum. In Proceedings of the Advanced Materials Research; Trans Tech Publications Ltd.: Bach, Switzerland, 2014; Volume 989–994, pp. 747–750. [Google Scholar]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 10537. [Google Scholar] [CrossRef] [Green Version]
- Kolb, N.; Vallorani, L.; Milanović, N.; Stocchi, V. Evaluation of Marine Algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata japonica) as Food Supplements. Food Technol. Biotechnol. 2004, 42, 57–61. [Google Scholar]
- Sugimura, R.; Suda, M.; Sho, A.; Takahashi, T.; Sashima, T.; Abe, M.; Hosokawa, M.; Miyashita, K. Stability of fucoxanthin in dried Undaria pinnatifida (wakame) and baked products (scones) containing wakame powder. Food Sci. Technol. Res. 2012, 18, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Kuramitsu, Y.; Kojoma, M.; Kim, S.Y.; Tanaka, T.; Maeda, H.; Miyashita, K.; Kawagoe, C.; Kohno, S.; Mutoh, M. High fucoxanthin wakame (Undaria pinnatifida) prevents tumor microenvironment formation in an AOM/DSS mouse carcinogenic model. J. Funct. Foods 2020, 64, 103709. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Sokovic, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Macroalgae as an Alternative Source of Nutrients and Compounds with Bioactive Potential. Proceedings 2020, 70, 46. [Google Scholar] [CrossRef]
- Terasaki, M.; Narayan, B.; Kamogawa, H.; Nomura, M.; Stephen, N.M.; Kawagoe, C.; Hosokawa, M.; Miyashita, K. Carotenoid profile of edible japanese seaweeds: An improved HPLC method for separation of major carotenoids. J. Aquat. Food Prod. Technol. 2012, 21, 468–479. [Google Scholar] [CrossRef]
- Honda, M.; Kodama, T.; Kageyama, H.; Hibino, T.; Wahyudiono; Kanda, H.; Goto, M. Enhanced Solubility and Reduced Crystallinity of Carotenoids, β-Carotene and Astaxanthin, by Z-Isomerization. Eur. J. Lipid Sci. Technol. 2018, 120, 1800191. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Wang, L.; He, L.; Wang, G. High light intensity increases the concentrations of β-carotene and zeaxanthin in marine red macroalgae. Algal Res. 2020, 47, 101852. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Jorge, G.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Ramluckan, K.; Moodley, K.G.; Bux, F. An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 2014, 116, 103–108. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, R.; Coutinho, J.A.P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Pinto, D.C.G.A.; Ventura, S.P.M. Recovery of pigments from Ulva rigida. Sep. Purif. Technol. 2021, 255, 117723. [Google Scholar] [CrossRef]
- Cvitković, D.; Dragović-Uzelac, V.; Dobrinčić, A.; Čož-Rakovac, R.; Balbino, S. The effect of solvent and extraction method on the recovery of lipid fraction from Adriatic Sea macroalgae. Algal Res. 2021, 56, 102291. [Google Scholar] [CrossRef]
- Martins, M.; Mesquita, L.M.D.S.; Vaz, B.M.C.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Quéguineur, B.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and Fractionation of Pigments from Saccharina latissima (Linnaeus, 2006) Using an Ionic Liquid + Oil + Water System. ACS Sustain. Chem. Eng. 2021, 9, 6599–6612. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of natural deep eutectic solvents for extraction of hydrophilic and lipophilic compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Hallerud, C.B. Pigment Composition of Macroalgae from a Norwegian Kelp Forest. 2014. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/238838 (accessed on 13 December 2021).
- MarLIN: The Marine Life Information Network. for Britain & Ireland. Available online: https://www.marlin.ac.uk/ (accessed on 13 December 2021).


| Algae | Solvent | Yield (%) | Chlorophylls | Xanthophylls | Carot. | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | Total | X1 | X2 | X3 | X4 | X5 | X6 | Total | B1 | ||||
| AN | EtOH | 18.15 | 20.6 | - | 34.4 | 31.6 | 35.6 | 23.1 | 145.3 | 623.9 | - | - | 132.1 | - | 2.6 | 758.7 | - | 904.0 |
| AcO | 15.38 | 64.2 | 17.6 | 170.8 | 343.8 | 21.8 | 30.1 | 648.2 | 940.9 | - | 8.7 | 201.0 | 8.6 | - | 1159.1 | 1085.4 | 2892.7 | |
| CHCl3 | 9.43 | 20.8 | 20.8 | 90.7 | 127.2 | - | 16.1 | 275.7 | 696.8 | 1.3 | 6.3 | 147.5 | 9.4 | - | 861.3 | - | 1137.0 | |
| EtAc | 10.31 | - | - | 61.5 | 106.5 | 19.7 | 20.4 | 208.1 | 697.2 | - | 6.8 | 147.6 | 0.4 | - | 852.0 | - | 1060.1 | |
| Hex | 7.99 | - | - | 32.7 | 42.9 | - | - | 75.6 | 673.4 | 129.9 | 21.9 | 40.0 | 17.0 | - | 882.2 | - | 957.8 | |
| BB | EtOH | 24.15 | 32.9 | 21.7 | - | - | - | 36.9 | 91.6 | 806.2 | - | - | 146.0 | - | 132.7 | 1084.9 | 3168.7 | 4345.2 |
| AcO | 10.85 | 37.8 | 28.7 | - | 357.1 | 68.0 | 36.0 | 527.6 | 623.0 | - | - | 187.2 | - | 107.7 | 917.8 | 4758.4 | 6203.8 | |
| CHCl3 | 4.43 | - | - | - | 210.8 | 35.7 | 27.9 | 274.3 | 631.9 | - | - | 164.6 | - | 100.6 | 897.0 | 1354.1 | 2525.4 | |
| EtAc | 4.43 | 28.4 | 22.3 | - | 323.4 | 45.9 | 54.4 | 474.4 | 736.3 | - | - | 186.5 | - | 130.6 | 1053.4 | 7261.5 | 8789.3 | |
| Hex | 1.57 | - | - | - | 167.3 | 50.4 | - | 217.7 | 271.5 | - | - | 50.5 | - | 79.0 | 401.0 | - | 618.7 | |
| FS | EtOH | 14.63 | 19.3 | - | - | - | - | 39.7 | 58.9 | 1171.3 | - | - | 233.9 | - | 5.2 | 1410.3 | - | 1469.2 |
| AcO | 7.75 | 79.7 | 49.6 | 212.0 | 471.5 | 23.3 | - | 836.1 | 1417.4 | - | 10.5 | 278.9 | 45.3 | 19.3 | 1771.5 | - | 2607.6 | |
| CHCl3 | 7.03 | - | - | - | 135.6 | 191.9 | 20.5 | 348.0 | 1547.7 | 13.3 | 23.3 | 276.4 | 26.1 | - | 1886.8 | - | 2234.8 | |
| EtAc | 5.31 | 55.7 | 22.6 | - | 97.6 | 162.4 | 19.0 | 357.2 | 1490.9 | 33.2 | 30.1 | 267.8 | 6.7 | - | 1828.8 | - | 2186.0 | |
| Hex | 5.75 | - | - | - | 45.8 | 66.1 | 22.9 | 134.8 | 1008.7 | 215.7 | 44.8 | 120.8 | - | 1.8 | 1391.7 | 446.3 | 1972.8 | |
| HE | EtOH | 26.99 | 500.1 | 176.3 | 35.6 | 1039.6 | 126.0 | 50.7 | 1928.3 | 3114.1 | - | 37.6 | 543.1 | 52.0 | 119.5 | 3866.4 | 6141.7 | 11,936.4 |
| AcO | 3.60 | 140.6 | 65.9 | 20.9 | 1308.2 | 105.4 | 32.1 | 1673.0 | 2985.5 | 163.8 | 60.1 | 512.9 | 38.2 | 116.4 | 3876.8 | 4328.7 | 9878.6 | |
| CHCl3 | 3.15 | - | - | - | 719.4 | 52.4 | 24.8 | 796.5 | 2771.3 | 109.4 | 30.3 | 517.3 | 44.9 | 106.0 | 3579.2 | 1670.3 | 6046.0 | |
| EtAc | 0.15 | 52.4 | 31.9 | - | 819.5 | 61.6 | 25.3 | 990.8 | 1765.5 | 57.3 | 55.3 | 346.9 | - | 65.2 | 2290.2 | 3186.3 | 6467.2 | |
| Hex | 2.10 | - | - | - | 352.1 | 111.5 | 33.0 | 496.6 | 1512.0 | 235.9 | 48.1 | 178.0 | 2.7 | 57.9 | 2034.6 | 2782.4 | 5313.6 | |
| LO | EtOH | 19.2 | 16.6 | 18.0 | 172.4 | 432.8 | 570.4 | 74.2 | 1284.4 | 2926.9 | - | - | 237.9 | 2.8 | - | 3167.6 | 4571.9 | 9023.9 |
| AcO | 0.8 | - | - | 25.7 | 1393.9 | 83.5 | - | 1503.1 | 2427.7 | 143.7 | 18.6 | 498.6 | 10.1 | - | 3098.7 | 5675.5 | 10277.3 | |
| CHCl3 | 2.1 | - | - | 55.7 | 398.2 | 28.0 | 16.9 | 498.8 | 697.3 | 51.2 | - | 236.5 | - | - | 985.0 | 470.2 | 1954.0 | |
| EtAc | 1.4 | - | - | 19.3 | 968.0 | 64.1 | 23.0 | 1074.4 | 1709.0 | 83.6 | 7.2 | 549.1 | - | - | 2349.0 | 1044.6 | 4468.0 | |
| Hex | 0.6 | - | - | 26.7 | 73.3 | 17.6 | - | 117.6 | 871.9 | 65.1 | - | 325.2 | - | - | 1262.2 | - | 1379.7 | |
| LS | EtOH | 17.97 | 35.5 | 69.8 | 628.8 | 604.5 | 131.8 | 64.9 | 1535.3 | 3904.8 | - | 69.9 | 687.5 | 5.6 | - | 4667.9 | 2895.7 | 9098.8 |
| AcO | 2.92 | 25.1 | 38.3 | 36.6 | 976.3 | 128.3 | 25.4 | 1229.9 | 1844.4 | 102.0 | 29.9 | 388.8 | - | - | 2365.1 | 2587.8 | 6182.8 | |
| CHCl3 | 1.69 | - | - | 72.9 | 585.0 | 123.6 | 36.3 | 817.9 | 3344.7 | 72.3 | 55.4 | 769.2 | - | 27.5 | 4269.0 | - | 5086.9 | |
| EtAc | 0.61 | - | - | 39.0 | 352.1 | 48.9 | 23.4 | 463.4 | 1264.0 | 10.7 | 17.4 | 416.3 | - | 5.0 | 1713.4 | 1202.3 | 3379.1 | |
| Hex | 1.38 | - | - | 30.8 | 51.6 | 28.2 | 20.8 | 131.4 | 670.7 | 58.4 | 3.7 | 256.9 | - | - | 989.6 | - | 1121.0 | |
| PC | EtOH | 15.63 | 53.6 | 33.0 | - | 199.3 | 28.8 | 22.2 | 336.9 | 717.6 | - | 11.1 | 137.2 | - | 0.4 | 866.3 | - | 1203.2 |
| AcO | 12.18 | 70.4 | 42.9 | 16.5 | 247.4 | 19.7 | 18.5 | 415.3 | 923.1 | 11.1 | 17.3 | 192.3 | 4.0 | 16.0 | 1163.9 | - | 1579.2 | |
| CHCl3 | 7.59 | - | - | - | 81.2 | 108.4 | 20.9 | 210.5 | 1487.1 | - | 0.1 | 241.1 | - | - | 1728.4 | - | 1938.9 | |
| EtAc | 7.74 | 22.6 | 19.7 | - | 226.6 | 16.6 | 17.9 | 303.3 | 643.6 | 7.5 | 11.1 | 137.1 | 1.8 | 10.7 | 811.8 | - | 1115.2 | |
| Hex | 6.87 | - | - | - | 115.8 | 27.3 | 17.2 | 160.4 | 815.1 | 309.7 | 35.0 | 119.2 | - | 15.5 | 1294.5 | - | 1454.8 | |
| SM | EtOH | 17.9 | 58.1 | 21.1 | - | - | - | 37.1 | 116.3 | 2322.5 | - | - | 437.1 | - | 9.3 | 2768.8 | - | 2885.1 |
| AcO | 3.4 | 184.4 | 116.1 | 313.6 | 828.0 | 51.1 | 62.0 | 1555.2 | 1735.4 | 23.2 | 9.5 | 381.9 | 32.2 | 18.2 | 2200.4 | 3930.9 | 7686.5 | |
| CHCl3 | 2.1 | - | - | 26.3 | 354.0 | 30.8 | 15.8 | 426.8 | 826.9 | 16.5 | - | 261.7 | - | - | 1105.1 | 851.7 | 2383.7 | |
| EtAc | 0.3 | 15.7 | 19.4 | 36.3 | 174.8 | 315.2 | - | 561.5 | 1591.8 | 6.8 | 2.8 | 359.7 | 1.5 | - | 1962.6 | - | 2524.1 | |
| Hex | 2.0 | - | - | 80.8 | 142.4 | 41.3 | - | 264.5 | 897.1 | 79.2 | 5.7 | 132.0 | - | - | 1113.9 | - | 1378.5 | |
| UP | EtOH | 38.84 | 504.3 | 346.7 | - | 33.7 | - | 26.7 | 911.3 | 2576.3 | - | 16.3 | 432.5 | 2.1 | 54.8 | 3082.0 | 2839.8 | 6833.2 |
| AcO | 3.35 | - | 35.2 | - | 773.0 | 49.0 | 26.7 | 883.9 | 3310.7 | - | 45.0 | 597.2 | - | - | 3952.8 | 2792.4 | 7629.1 | |
| CHCl3 | 3.22 | 55.8 | 56.6 | 174.3 | 249.0 | - | 24.7 | 560.4 | 3777.8 | - | 3.8 | 707.3 | 4.7 | 21.8 | 4515.4 | - | 5075.8 | |
| EtAc | 2.38 | 35.6 | 39.6 | - | 624.1 | 34.5 | 36.0 | 769.9 | 2536.2 | - | 26.7 | 545.5 | - | 35.1 | 3143.5 | 3147.7 | 7061.1 | |
| Hex | 2.24 | - | - | - | 38.6 | - | 30.2 | 68.7 | 1705.3 | - | 28.5 | 212.2 | 29.9 | 28.6 | 2004.5 | 1639.3 | 3712.5 | |
| HPLC parameters | RT (min) | 9.88 | 10.5 | 32.1 | 32.4 | 32.7 | 34.2 | - | 16.8 | 19.5 | 21.4 | 22.5 | 24.2 | 26.8 | - | 34.6 | - | |
| λ max (nm) | 452, 584, 634 | 448, 580, 632 | 430, 616, 662 | 430, 616, 664 | 430, 618, 664 | 410, 608, 666 | - | 450, 658 | 418, 440, 470 | 382, 402, 428 | 442, 658 | 402, 426, 456, 658 | (424), 452, 478 | - | (416), 450, 476 | - | ||
| Species 2 | Extraction Solvent 3 // Determination 4 | Compounds 5 | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Chl c | Chl a | Fx | Vx | Bcar | |||
| AN | Several // HPLC-DAD | 1.0 | 0.66 | 0.13 | 0.1 | [49] | |
| Several // HPLC-DAD | 1.78 | [50] | |||||
| AcO:water (9:1, v:v) // HPLC-DAD | 1.34 | [6] | |||||
| AcO:water (9:1, v:v) // HPLC-DAD | 3.66 | 1.8 | 0.5 | [37] | |||
| AcO:water (9:1, v:v) // HPLC-DAD | 0.3 | 1.5 | 0.7 | 1.1 | [35] | ||
| AcO // HPLC-DAD | 0.4 | [51] | |||||
| FS | MeOH // UV-Vis | 3.0 | [52] | ||||
| MeOH:Hex (1:4, v:v) // UV-Vis | 0.0005 * | 0.171 * | 0.007 * | [53] | |||
| MeOH // UV-Vis | 0.171 * | [54] | |||||
| HE | MeOH // UV-Vis | 0.63 | [32] | ||||
| MeOH// UV-Vis | 0.60 | [32] | |||||
| EtOH // UV-Vis | 0.68 | [32] | |||||
| AcO // UV-Vis | 1.57 | [32] | |||||
| DMSO:water (4:1, v:v) // UV-Vis | 0.28 | [32] | |||||
| Hex:EtOEt:CHCl3 (1:1:1, v:v:v) // LC-DAD-ESI-MS and NMR | 18.60 | [41] | |||||
| AcO:water (9:1, v:v) // HPLC-DAD | 2.8 | 3.3 | 0.58 | [37] | |||
| AcO // HPLC–DAD | 0.05 * | [47] | |||||
| AcO:water (9:1, v:v) // HPLC-DAD | 0.4 | 1.6 | 1.1 | 1.5 | [35] | ||
| MeOH:Hex:CH2Cl2 (50:25:25, v:v:v) // HPLC-MS | 1.5 | 0.009 | 0.004 | [45] | |||
| MeOH:Hex: CH2Cl2 (50:25:25, v:v:v) // HPLC-DAD | 0.043 | 0.051 | 0.0095 | [46] | |||
| AcO // HPLC-DAD | 0.3 | [51] | |||||
| LO | DMF // HPLC-DAD | 0.08 * | 0.36 * | 0.01 * | [38] | ||
| AcO // HPLC-DAD | 0.018 | 0.163 | 0.006 | 0.005 | [37] | ||
| LS | MeOH // HPLC-DAD | 0.183 | [55] | ||||
| MeOH // HPLC-DAD | 0.655 | 0.665 | 0.036 | 0.023 | [42] | ||
| MeOH // UV-Vis | 0.143 | [32] | |||||
| MeOH// UV-Vis | 0.111 | [32] | |||||
| EtOH // UV-Vis | 0.114 | [32] | |||||
| AcO:water (9:1, v:v) // HPLC-DAD | 1.35 | 0.59 | 0.02 | 0.03 | [56] | ||
| AcO // HPLC–DAD | 0.016 | [47] | |||||
| AcO:water (9:1, v:v) // HPLC-DAD | 0.09 | 0.08 | [57] | ||||
| AcO // UV-Vis | 0.184 | [32] | |||||
| AcO // HPLC-DAD | 0.029 | 0.433 | 0.302 | 0.316 | [43] | ||
| AcO:water (7:3, v:v) // UV-Vis | 0.38 | 0.58 | [58] | ||||
| PC | Hex // UV-Vis | 0.602 | 0.236 | [59] | |||
| AcO // UV-Vis | 1.2 * | [60] | |||||
| SM | AcO // UV-Vis | 0.440 | 2.720 | 0.080 | [36] | ||
| AcO // UV-Vis | 0.4 | [61] | |||||
| AcO:water (8:2, v:v) // UV-Vis | 2.1 * | [62] | |||||
| UP | MeOH // UV-Vis | 0.349 | [32] | ||||
| MeOH // UV-Vis | 0.331 | [32] | |||||
| MeOH // HPLC-DAD | 0.013 | [63] | |||||
| MeOH // HPLC-DAD | 0.728 | [64] | |||||
| MeOH // HPLC-DAD | 5.0 | [65] | |||||
| EtOH // UV-Vis | 0.321 | [32] | |||||
| EtOH:water (8:2, v:v) // UV-Vis | 0.008 | [66] | |||||
| EtOH:water (3:1, v:v) // HPLC-DAD | 3.37 | [40] | |||||
| AcO // UV-Vis | 0.543 | [32] | |||||
| AcO // HPLC-DAD | 2.3 | [67] | |||||
| DMF // UV-Vis | 0.437 | [32] | |||||
| DMSO:water (4:1, v:v) // UV-Vis | 0.014 | [32] | |||||
| Water // HPLC-DAD | 0.73 | [44] | |||||
| EtOEt // HPLC-DAD | 0.39 * | [48] | |||||
| Species | Family | Common Name(s) | Distribution | Depth | Habitat Preferences |
|---|---|---|---|---|---|
| Ascophyllum nudosum | Fucaceae | Rockweed, Norwegian kelp, Egg wrack | North Atlantic Ocean | Not relevant | Sheltered rocky shores, intertidal habit. |
| Bifurcaria bifurcata | Fucaceae | Brown Tuning Fork Weed, Brown Forking Weed | From Ireland to Senegal. | Ponds | Rock pools on the middle and lower shore, particularly on exposed beaches. It also forms a low water zone in some locations in Southwest England and West Ireland. |
| Fucus spiralis | Fucaceae | Spiral wrack, Flat wrack | North Atlantic Ocean and isolated reports in the Northern Pacific. | Not relevant | Rocky substrata on sheltered to moderately exposed shores, intertidal habit. |
| Himanthalia elongata | Fucaceae | Sea spaghetti | From Norway to Portugal | Intertidal and infralittoral zone | Forming a very characteristic band |
| Laminaria saccharina | Laminariaceae | Sugar kelp, Sea belt, Devil’s apron | Arctic Ocean down to Northern Portugal | Sublittoral zone (max 30 m) | Moderately to sheltered sites, often on unstable substrate (boulders, mussels and rocks) |
| Laminaria orcholeuca | Laminariaceae | Kelp | Arctic and Atlantic Oceans | Lower littoral and sublittoral zone (max 20 m) | Exposed to moderately exposed sites, hard substrate and strong currents |
| Pelvetia canaliculata | Fucaceae | Channelled wrack, Cow tang | European coastline | Supralittoral zone | Sheltered to moderately exposed, hard substrate and high tolerance of desiccation |
| Sargassum muticum | Fucaceae | Japanese wireweed | Atlantic and Pacific Oceans | - | Hard substrata in shallow waters |
| Undaria pinnatifida | Laminariaceae | Wakame | Northern Europe, Argentina, Mexico, Australia, New Zealand, Japan, Korea and China | Infralittoral | Grows on stones, epiphyte or artificial structures (swamps and ship hulls) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Perez, P.; Lourenço-Lopes, C.; Silva, A.; Pereira, A.G.; Fraga-Corral, M.; Zhao, C.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Pigment Composition of Nine Brown Algae from the Iberian Northwestern Coastline: Influence of the Extraction Solvent. Mar. Drugs 2022, 20, 113. https://0-doi-org.brum.beds.ac.uk/10.3390/md20020113
Garcia-Perez P, Lourenço-Lopes C, Silva A, Pereira AG, Fraga-Corral M, Zhao C, Xiao J, Simal-Gandara J, Prieto MA. Pigment Composition of Nine Brown Algae from the Iberian Northwestern Coastline: Influence of the Extraction Solvent. Marine Drugs. 2022; 20(2):113. https://0-doi-org.brum.beds.ac.uk/10.3390/md20020113
Chicago/Turabian StyleGarcia-Perez, Pascual, Catarina Lourenço-Lopes, Aurora Silva, Antia G. Pereira, Maria Fraga-Corral, Chao Zhao, Jianbo Xiao, Jesus Simal-Gandara, and Miguel A. Prieto. 2022. "Pigment Composition of Nine Brown Algae from the Iberian Northwestern Coastline: Influence of the Extraction Solvent" Marine Drugs 20, no. 2: 113. https://0-doi-org.brum.beds.ac.uk/10.3390/md20020113