Bioactivity-Guided Screening of Antimicrobial Secondary Metabolites from Antarctic Cultivable Fungus Acrostalagmus luteoalbus CH-6 Combined with Molecular Networking
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Antimicrobial Screening of Soil-Derived Fungi from Fildes Peninsula, Antarctica
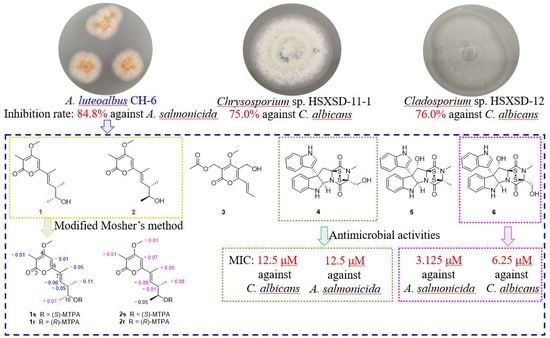
2.2. Identification of the Bioactive Fungi
2.3. Secondary Metabolites Profile Visualization and Annotation by Molecular Networking
2.4. Structure Elucidations of Isolated Compounds 1–6
2.5. Antifungal and Antibacterial Activity Evaluations of Isolated Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation and Fermentation of Soil-Derived Fungi from the Fildes Peninsula, Antarctica
3.3. Extraction and Bioactivities Screening of Fermented Fungi
3.4. The Identification of the Bioactive Fungal Strains
3.5. Molecular Networking
3.5.1. UHPLC Parameters
3.5.2. MS2 Parameters
3.5.3. Molecular Network Analysis
3.6. Extraction and Isolation of Compounds 1–6 from Acrostalagmus luteoalbus CH-6
3.7. Preparation of the (S)- and (R)-MTPA Esters of 1 and 2
3.8. Antibacterial and Antifungal Activity Evaluations of the Isolated Compounds 1–6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust and HM Government: London, UK, 2016. [Google Scholar]
- Monowar, T.; Rahman, M.S.; Bhore, S.J.; Sathasivam, K.V. Endophytic bacteria Enterobacter hormaechei fabricated silver nanoparticles and their antimicrobial activity. Pharmaceutics 2021, 13, 511. [Google Scholar] [CrossRef] [PubMed]
- Lomazzi, M.; Moore, M.; Johnson, A.; Balasegaram, M.; Borisch, B. Antimicrobial resistance–moving forward? BMC Public Health 2019, 19, 858–863. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; World Health Organization: Geneva, Switzerland, 2021; License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- CDC. Antibiotic Resistance Threats in the United States; Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- WHO. 2019 Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; World Health Organization: Geneva, Switzerland, 2019; License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Butler, M.S.; Buss, A.D. Natural products–the future scaffolds for novel antibiotics? Biochem. Pharmacol. 2006, 71, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Current challenges in the discovery of novel antibacterials from microbial natural products. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Yin, X.; Deng, A.; Shen, J.; Tian, Y.; Wang, S.; Yang, H. Diversity of cultivable microbes from soil of the Fildes Peninsula, Antarctica, and their potential application. Front. Microbiol. 2020, 11, 570836. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Sun, C.; Zhang, G.; Che, Q.; Li, D. Antibacterial polyketides from Antarctica sponge-derived fungus Penicillium sp. HDN151272. Mar. Drugs 2020, 18, 71. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, Z.; Ren, Z.; Yu, L.; Zhu, T. Antibacterial cyclic tripeptides from Antarctica-sponge-derived fungus Aspergillus insulicola HDN151418. Mar. Drugs 2020, 18, 532. [Google Scholar] [CrossRef]
- Shi, T.; Yu, Y.-Y.; Dai, J.-J.; Zhang, Y.-T.; Hu, W.-P.; Zheng, L.; Shi, D.-Y. New polyketides from the Antarctic fungus Pseudogymnoascus sp. HSX2#-11. Mar. Drugs 2021, 19, 168. [Google Scholar]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P. Structure of a C17 antifungal terpenoid from an unidentified Acrostalagmus species. J. Amer. Chem. Soc. 1969, 91, 2134–2136. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P.; Lancaster, J.E.; Morton, G.O. Structure and chemistry of antibiotic LL-Z1271α, an antifungal carbon-17 terpene. J. Amer. Chem. Soc. 1970, 92, 5483–5489. [Google Scholar] [CrossRef]
- Argoudelis, A.D.; Reusser, F. Melinacidins, a new family of antibiotics. J. Antibiot. 1971, 24, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Argoudelis, A.D. Melinacidins II, III, and IV. New 3,6-epidithiadiketopiperazine antibiotics. J. Antibiot. 1972, 25, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kakisawa, H. Structures of three new C16 terpenoids from an Acrostalagmus fungus. J. Chem. Soc. Perkin Trans. 1976, 1, 2407–2413. [Google Scholar] [CrossRef]
- Rojas, N.L.; Voget, C.E.; Hours, R.A.; Cavalitto, S.F. Purification and characterization of a novel alkaline α-L-rhamnosidase produced by Acrostalagmus luteoalbus. J. Ind. Microbiol. Biotechnol. 2011, 38, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Z.; Huang, Z.; Shi, X.-F.; Chen, Y.-C.; Zhang, W.-M.; Tian, X.-P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus Acrostalagmus luteoalbus HDN13-530. RSC Adv. 2018, 8, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Li, X.-M.; Meng, L.-H.; Konuklugil, B.; Li, X.; Li, H.-L.; Wang, B.-G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019, 90, 103030. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.-M.; Li, X.; Li, H.-L.; Konuklugil, B.; Wang, B.-G. Uncommon N-methoxyindolediketopiperazines from Acrostalagmus luteoalbus, a marine algal isolate of endophytic fungus. Chin. J. Chem. 2021, 39, 2808–2814. [Google Scholar] [CrossRef]
- Ramos, A.E.F.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural products targeting strategies involving molecular networking: Different manners, one goal. Nat. Prod. Rep. 2019, 36, 960–980. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Hou, X.M.; Li, Y.Y.; Shi, Y.W.; Fang, Y.W.; Shao, C.L. Integrating molecular networking and 1H NMR to target the isolation of chrysogeamides from a library of marine-derived Penicillium fungi. J. Org. Chem. 2019, 84, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the Antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Cherblanc, F.; Lo, Y.-P.; De Gussem, E.; Alcazar-Fuoli, L.; Bignell, E.; He, Y.; Chapman-Rothe, N.; Bultinck, P.; Herrebout, W.A.; Brown, R.; et al. On the determination of the stereochemistry of semisynthetic natural product analogues using chiroptical spectroscopy: Desulfurization of epidithiodioxopiperazine fungal metabolites. Chem. Eur. J. 2011, 17, 11868–11875. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Suzuki, Y.; Koyama, K.; Natori, S.; Iitaka, Y.; Kinosita, T. Chetracin A and chaetocins B and C, three new epipolythiodioxo-piperazines from Chaetomium spp. Chem. Pharm. Bull. 1988, 36, 1942–1956. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Koyama, K.; Natori, S.; Iitaka, Y. Chetracin A, a new epipolythiodioxopiperazine having a tetrasulfide bridge from Chaetomium abuense and C. retardatum. Tetrahedron Lett. 1985, 26, 4731–4734. [Google Scholar] [CrossRef]
- Minato, H.; Matsumoto, M.; Katayama, T. Studies on the metabolites of Verticillium sp. Structures of verticillins A, B, and C. J. Chem. Soc. Perkin. Trans. 1973, 1, 1819–1825. [Google Scholar] [CrossRef]
- Byeng, W.S.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. New cytotoxic epidithiodioxopiperazines related to verticillin A from a marine isolate of the fungus Penicillium. Nat. Prod. Lett. 1999, 13, 213–222. [Google Scholar]
- Dong, J.-Y.; He, H.-P.; Shen, Y.-M.; Zhang, K.-Q. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J. Nat. Prod. 2005, 68, 1510–1513. [Google Scholar] [CrossRef]
- Feng, Y.; Blunt, J.W.; Cole, A.L.; Munro, M.H. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. J. Nat. Prod. 2004, 67, 2090–2092. [Google Scholar] [CrossRef]
- Proksch, P.; Ebel, R.; Edrada, R.; Riebe, F.; Liu, H.; Diesel, A.; Bayer, M.; Li, X.; Lin, W.H.; Grebenyuk, V.; et al. Sponge-associated fungi and their bioactive compounds: The Suberites case. Bot. Mar. 2008, 51, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, X.-M.; Meng, L.; Li, C.-S.; Gao, S.-S.; Shang, Z.; Proksch, P.; Huang, C.-G.; Wang, B.-G. Nigerapyrones A–H, α-pyrone derivatives from the marine mangrove-derived endophytic fungus Aspergillus niger MA-132. J. Nat. Prod. 2011, 74, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Khamthong, N.; Sukpondma, Y.; Phongpaichit, S.; Hutadilok-Towatana, N.; Graidist, P.; Sakayaroj, J.; Kirtikara, K. Cyclohexene, diketopiperazine, lactone and phenol derivatives from the sea fan-derived fungi Nigrospora sp. PSU-F11 and PSU-F12. Arch. Pharmacol. Res. 2010, 33, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wen, Y.; Cheng, H.; Deng, J.; Peng, Y.; Bahetejiang, Y.; Huang, H.; Wu, C.; Yang, X.; Pang, K. Penpolonin A–E, cytotoxic α-pyrone derivatives from Penicillium polonicum. Bioorg. Med. Chem. Lett. 2021, 40, 127921. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Parisi, A.; Piattelli, M.; Magnano Di San Lio, G. Phomenins A and B, bioactive polypropionate pyrones from culture fluids of Phoma tracheiphila. Nat. Prod. Lett. 1993, 3, 101–106. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Chumala, P.B. Phomapyrones from blackleg causing phytopathogenic fungi: Isolation, structure determination, biosyntheses and biological activity. Phytochemistry 2005, 66, 81–87. [Google Scholar] [CrossRef]
- Kusumi, T.; Ohtani, I.; Inouye, Y.; Kakisawa, H. Absolute configurations of cytotoxic marine cembranolides; consideration of Mosher’s method. Tetrahedron Lett. 1988, 29, 4731–4734. [Google Scholar] [CrossRef]
- Li, F.; Ye, Z.; Huang, Z.; Chen, X.; Sun, W.; Gao, W.; Zhang, S.; Cao, F.; Wang, J.; Hu, Z.; et al. New α-pyrone derivatives with herbicidal activity from the endophytic fungus Alternaria brassicicola. Bioorg. Chem. 2021, 117, 105452. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Nagano, J.; Natori, H.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from three ascomycetes, Gelasinospora heterospora, G. multiforis, and G. longispora. Chem. Pharm. Bull. 1999, 47, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Adams, T.C.; Payette, J.N.; Cheah, J.H.; Movassaghi, M. Concise total synthesis of (+)-luteoalbusins A and B. Org. Lett. 2015, 17, 4268–4271. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Li, X.-Q.; Zheng, L.; Zhang, Y.-T.; Dai, J.-J.; Shang, E.-L.; Yu, Y.-Y.; Zhang, Y.-H.; Hu, W.-P.; Shi, D.-Y. Sesquiterpenoids from the Antarctic fungus Pseudogymnoascus sp. HSX2#-11. Front. Microbiol. 2021, 12, 688202. [Google Scholar]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Qian, P.-Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2009, 26, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabhapathy, M.; Nagata, S. Cross-species induction of antibacterial activity produced by epibiotic bacteria isolated from Indian marine sponge Pseudoceratina purpurea. World J. Microbiol. Biotechnol. 2008, 24, 687–691. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Bandeira, N. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Strain Number | Inhibition Rate % a (Staticity/Shaking) | ||||
|---|---|---|---|---|---|
| C. albicans | E. coli | S. aureus | B. subtilis | P. aeruginosa | |
| HSXSD-11-1 | 75.0 ± 3.9/71.4 ± 0.7 | –/15.2 ± 4.2 | 41.4 ± 2.6/40.3 ± 1.8 | 13.6 ± 0.5/18.0 ± 4.6 | –/– |
| HSXSD-12 | 76.0 ± 3.1/– | –/– | –/25.1 ± 0.6 | –/12.9 ± 2.8 | –/– |
| HSX2#-13 | –/– | –/– | –/– | –/16.6 ± 2.5 | –/– |
| HSX6#-16 | –/18.9 ± 4.1 | –/– | –/24.0 ± 1.5 | 15.7 ± 4.8/21.4 ± 1.0 | 10.4 ± 2.4/11.9 ± 4.2 |
| CH-6 | 78.7 ± 1.2/72.3 ± 0.8 | –/– | 39.3 ± 3.7/49.6 ± 4.1 | 19.2 ± 2.4/17.8 ± 0.3 | 12.8 ± 4.0/16.1 ± 4.6 |
| CH-9 | –/– | –/– | –/– | 12.2 ± 2.1/17.1 ± 0.5 | –/– |
| DLW-10 | –/12.2 ± 4.9 | 17.1 ± 1.3/17.6 ± 3.0 | 20.6 ± 4.5/24.4 ± 1.1 | –/12.3 ± 2.8 | –/– |
| WLG-10 | –/– | 21.5 ± 2.7/– | 16.9 ± 2.3/– | –/– | –/– |
| HSXSD-6 | –/– | –/14.1 ± 2.4 | –/– | –/12.8 ± 3.2 | –/– |
| HSXSD-10 | –/– | 13.8 ± 1.0/16.6 ± 1.9 | –/22.5 ± 0.1 | –/15.3 ± 0.8 | –/– |
| HSX2#-5 | –/– | 22.2 ± 2.4/– | –/16.4 ± 0.7 | –/18.7 ± 1.3 | –/12.3 ± 2.8 |
| HSX2#-7 | –/– | 10.2 ± 2.6/– | –/13.8 ± 1.5 | –/14.4 ± 1.7 | –/17.1 ± 2.0 |
| HSX2#-11 | 29.6 ± 4.5/14.3 ± 3.6 | –/– | 19.6 ± 3.5/27.3 ± 0.2 | 12.9 ± 1.3/18.2 ± 1.2 | –/– |
| HSX2#-12 | 23.2 ± 2.4/22.7 ± 1.8 | –/– | 18.6 ± 0.5/27.9 ± ±0.1 | 14.7 ± 5.1/– | –/– |
| HSX2#-15 | 21.7 ± 6.8/– | –/– | –/12.8 ± 2.1 | 15.1 ± 4.5/11.5 ± 0.7 | –/– |
| HSX11#-3 | 24.7 ± 4.9/– | –/12.6 ± 9.5 | –/22.1 ± 0.8 | 16.1 ± 2.5/– | –/– |
| HSX11#-4 | 20.5 ± 3.2/– | –/– | –/– | 12.8 ± 0.5/15.2 ± 1.6 | –/– |
| HSX6#-8 | –/– | 11.7 ± 4.0/14.4 ± 0.8 | –/26.4 ± 2.6 | 10.2 ± 1.1/– | –/– |
| HSX6#-10 | 30.3 ± 2.0/– | 23.1 ± 2.2/– | 20.8 ± 1.4/25.5 ± 0.0 | 17.0 ± 3.5/15.3 ± 0.9 | –/– |
| HSX6#-14 | 16.1 ± 5.4/– | 11.1 ± 2.8/16.0 ± 2.9 | 24.4 ± 5.0/– | –/– | –/– |
| HSX8#-6 | 20.6 ± 1.4/– | –/13.7 ± 0.1 | 22.2 ± 2.7/10.9 ± 8.8 | 16.3 ± 1.9/– | –/– |
| HSX8#-9 | 15.9 ± 2.5/– | 11.1 ± 3.8/– | 21.9 ± 0.1/– | –/– | –/– |
| JQW-6 | 24.4 ± 4.7/– | 11.0 ± 1.1/– | –/– | 19.4 ± 2.5/– | –/– |
| JQW-8 | 18.3 ± 4.4/– | –/– | 10.5 ± 1.0/– | 21.3 ± 2.3/12.7 ± 3.2 | –/– |
| WLG-7 | 11.1 ± 1.4/– | –/– | –/– | 11.3 ± 2.2/– | –/– |
| DLW-7 | –/– | –/– | –/– | –/– | –/– |
| CH-6-rice | 80.5 ± 0.9 | – | 48.2 ± 2.5 | 19.8 ± 1.6 | 18.1 ± 3.1 |
| CPFX (10 μM) | 66.2 ± 1.8 | 76.5 ± 2.3 | 79.1 ± 1.1 | 81.9 ± 3.4 | 70.2 ± 3.3 |
| Strain Number | Inhibition Rate % a (Staticity/Shaking) | ||||
|---|---|---|---|---|---|
| A. salmonicida | V. anguillarum | P. angustum | P. aeruginosa | P. halotolerans | |
| HSXSD-11-1 | –/– | 26.4 ± 2.6/32.0 ± 1.5 | 16.8 ± 2.3/14.6 ± 1.0 | 18.4 ± 4.5/14.9 ± 3.7 | –/– |
| HSXSD-12 | –/– | 29.6 ± 3.5/– | 16.1 ± 1.3/10.1 ± 2.0 | 12.1 ± 0.6/– | –/– |
| HSX2#-13 | –/– | –/11.4 ± 2.9 | 16.8 ± 1.6/– | 10.7 ± 0.6/– | –/– |
| HSX6#-16 | –/– | 24.5 ± 3.6/24.4 ± 0.5 | 17.5 ± 0.7/14.5 ± 1.1 | –/– | –/– |
| CH-6 | 84.8 ± 1.4/77.6 ± 1.0 | 50.6 ± 2.6/58.3 ± 3.1 | 12.9 ± 0.8/14.4 ± 0.4 | 22.1 ± 2.5/13.4 ± 4.0 | –/– |
| CH-9 | –/– | 10.9 ± 2.2/10.2 ± 0.2 | 13.2 ± 0.3/11.8 ± 0.8 | –/– | –/– |
| DLW-10 | –/– | –/20.8 ± 2.9 | –/– | –/– | –/– |
| WLG-10 | –/– | –/– | 10.8 ± 0.5/– | 16.8 ± 0.1/– | –/– |
| HSXSD-6 | –/– | –/– | 13.1 ± 1.7/– | –/– | –/– |
| HSXSD-10 | –/– | –/– | –/– | –/– | –/– |
| HSX2#-5 | –/– | –/– | –/– | –/– | –/– |
| HSX2#-7 | –/– | –/– | 12.3 ± 0.8/– | –/– | –/– |
| HSX2#-11 | 33.4 ± 2.5/13.3 ± 4.1 | –/– | 13.7 ± 1.2/– | –/– | –/– |
| HSX2#-12 | –/17.4 ± 3.8 | 18.3 ± 0.8/– | 13.7 ± 1.0/– | –/– | –/– |
| HSX2#-15 | –/13.9 ± 3.8 | 19.2 ± 0.7/– | 14.0 ± 0.1/– | –/– | –/– |
| HSX11#-3 | –/– | 23.8 ± 0.6/– | 10.6 ± 1.0/– | –/– | –/– |
| HSX11#-4 | –/17.9 ± 0.7 | –/– | –/– | –/– | –/– |
| HSX6#-8 | –/– | –/– | –/– | –/– | –/– |
| HSX6#-10 | –/– | 12.7 ± 1.5/– | –/– | –/– | –/– |
| HSX6#-14 | –/– | –/– | –/14.3 ± 2.9 | –/– | –/– |
| HSX8#-6 | –/22.3 ± 4.5 | –/– | –/14.5 ± 5.2 | –/– | –/– |
| HSX8#-9 | –/– | –/– | –/16.7 ± 3.0 | –/– | –/– |
| JQW-6 | 13.6 ± 2.4/– | 21.5 ± 3.2/– | –/16.7 ± 2.4 | –/– | –/– |
| JQW-8 | 14.6 ± 2.4/– | 18.2 ± 0.6/– | 10.6 ± 1.5/13.3 ± 0.8 | –/– | –/– |
| WLG-7 | 21.8 ± 2.1/– | 12.8 ± 4.1/– | 10.2 ± 0.5/12.4 ± 2.7 | –/– | –/– |
| DLW-7 | –/– | –/– | –/11.7 ± 0.9 | –/– | –/– |
| CH-6-rice | 86.7 ± 1.1 | 57.1 ± 2.4 | 12.2 ± 3.2 | 20.9 ± 3.1 | – |
| Sea-nine 211 (10 μM) | 78.1 ± 3.7 | 71.0 ± 2.1 | 81.5 ± 2.9 | 69.1 ± 2.1 | 67.5 ± 4.5 |
| Scheme Number | Inhibition Rate % a (Staticity/Shaking) | ||||
|---|---|---|---|---|---|
| E. cloacae | E. hormaechei | P. fulva | V. harveyi | A. hydrophila | |
| HSXSD-11-1 | –/– | 12.0 ± 1.9/– | –/– | 15.5 ± 1.8/12.6 ± 1.3 | –/– |
| HSXSD-12 | –/– | –/– | 10.1 ± 2.2/– | 16.2 ± 3.6/10.3 ± 2.9 | –/– |
| HSX2#-13 | 10.5 ± 0.7/19.1 ± 0.7 | 10.3 ± 0.3/12.9 ± 0.5 | –/12.8 ± 0.8 | 16.9 ± 3.2/11.7 ± 3.9 | –/12.8 ± 0.7 |
| HSX6#-16 | 13.3 ± 1.6/14.0 ± 1.8 | 11.6 ± 1.0/– | 14.3 ± 1.8/10.4 ± 0.8 | 18.6 ± 1.6/10.5 ± 2.3 | –/– |
| CH-6 | –/– | 10.1 ± 0.6/15.4 ± 0.8 | 11.4 ± 2.2/13.3 ± 1.2 | 20.0 ± 0.6/21.0 ± 0.9 | 19.4 ± 3.5/14.3 ± 2.3 |
| CH-9 | 21.1 ± 3.2/21.0 ± 0.4 | 11.5 ± 2.3/14.1 ± 0.3 | 12.2 ± 3.2/18.8 ± 1.1 | 13.1 ± 3.5/15.5 ± 1.9 | –/13.5 ± 1.6 |
| DLW-10 | –/– | –/– | –/11.9 ± 2.2 | 13.9 ± 1.8/15.5 ± 2.1 | –/– |
| WLG-10 | –/– | –/– | –/– | –/– | –/– |
| HSXSD-6 | 13.1 ± 7.5/– | –/– | –/– | 13.4 ± 1.5/10.7 ± 0.7 | –/– |
| HSXSD-10 | –/– | –/– | –/– | –/11.2 ± 1.7 | –/– |
| HSX2#-5 | –/10.1 ± 1.0 | –/– | –/– | –/10.1 ± 1.2 | –/– |
| HSX2#-7 | –/– | –/– | –/– | –/10.8 ± 1.8 | –/– |
| HSX2#-11 | –/– | –/– | –/– | 10.8 ± 3.2/– | –/– |
| HSX2#-12 | –/– | –/– | –/– | 12.4 ± 2.9/13.3 ± 1.7 | –/– |
| HSX2#-15 | 10.3 ± 4.2/– | –/– | –/– | 15.3 ± 1.4/13.3 ± 1.5 | –/– |
| HSX11#-3 | 15.3 ± 0.5/– | –/– | –/– | 15.2 ± 1.4/– | –/– |
| HSX11#-4 | 19.0 ± 0.6/– | 10.7 ± 0.6/– | 10.2 ± 2.6/– | 12.6 ± 1.3/11.0 ± 2.9 | 11.9 ± 1.1/– |
| HSX6#-8 | –/– | –/– | –/– | –/– | –/– |
| HSX6#-10 | –/– | –/– | –/– | –/11.2 ± 2.8 | –/– |
| HSX6#-14 | –/– | –/11.1 ± 0.7 | –/– | –/– | –/– |
| HSX8#-6 | –/– | –/– | –/– | –/13.2 ± 2.8 | –/– |
| HSX8#-9 | –/– | –/11.3 ± 1.2 | –/10.6 ± 1.9 | –/14.4 ± 4.3 | –/– |
| JQW-6 | 13.7 ± 0.8/– | –/11.9 ± 0.4 | –/10.1 ± 1.1 | –/16.0 ± 4.0 | –/– |
| JQW-8 | 19.1 ± 1.0/– | 10.2 ± 0.7/10.5 ± 1.2 | –/– | 15.0 ± 1.4/15.9 ± 1.2 | 11.5 ± 0.5/– |
| WLG-7 | 19.8 ± 0.7/– | –/– | –/– | 15.1 ± 1.8/13.7 ± 3.7 | 12.0 ± 0.4/– |
| DLW-7 | –/15.2 ± 2.9 | –/– | –/– | –/14.5 ± 2.5 | –/– |
| CH-6-rice | – | 12.7 ± 1.1 | 13.6 ± 1.8 | 22.3 ± 1.2 | 17.1 ± 2.8 |
| Sea-nine 211 (10 μM) | 70.4 ± 2.5 | 76.3 ± 4.6 | 81.1 ± 3.2 | 83.1 ± 2.5 | 69.7 ± 4.0 |
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 2 | 165.2, C | 165.2, C | ||
| 3 | 102.5, C | 102.6, C | ||
| 4 | 166.0, C | 166.0, C | ||
| 5 | 92.4, CH | 6.13, s | 92.5, CH | 6.15, s |
| 6 | 159.8, C | 159.7, C | ||
| 7 | 126.4, C | 125.7, C | ||
| 8 | 136.8, CH | 6.46, dd (10.3, 1.5) | 137.0, CH | 6.50, dd (10.1, 1.4) |
| 9 | 41.1, CH | 2.60, dp (10.3, 6.7) | 41.4, CH | 2.57, dp (10.1, 6.8) |
| 10 | 71.9, CH | 3.72, p (6.4) | 71.7, CH | 3.69, p (6.3) |
| 11 | 21.3, CH3 | 1.17, d (6.4) | 20.8, CH3 | 1.22, d (6.3) |
| 3-Me | 8.8, CH3 | 1.94, s | 8.8, CH3 | 1.938, s |
| 4-OMe | 56.3, CH3 | 3.91, s | 56.3, CH3 | 3.91, s |
| 7-Me | 13.1, CH3 | 1.94, s | 13.1, CH3 | 1.943, d (1.4) |
| 9-Me | 16.5, CH3 | 1.09, d (6.7) | 16.7, CH3 | 1.04, d (6.8) |
| Strains | C. albicans | A. salmonicida | P. halotolerans | P. fulva | S. aureus |
|---|---|---|---|---|---|
| 1 | >50 | >50 | >50 | >50 | >50 |
| 2 | >50 | >50 | >50 | >50 | >50 |
| 3 | >50 | >50 | >50 | >50 | >50 |
| 4 | 12.5 | 12.5 | >50 | >50 | >50 |
| 5 | 25 | 50 | >50 | >50 | >50 |
| 6 | 6.25 | 3.125 | 25 | 25 | 25 |
| CPFX | 6.25 | 6.25 | 0.195 | 1.56 | 3.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Li, X.-Q.; Wang, Z.-M.; Zheng, L.; Yu, Y.-Y.; Dai, J.-J.; Shi, D.-Y. Bioactivity-Guided Screening of Antimicrobial Secondary Metabolites from Antarctic Cultivable Fungus Acrostalagmus luteoalbus CH-6 Combined with Molecular Networking. Mar. Drugs 2022, 20, 334. https://0-doi-org.brum.beds.ac.uk/10.3390/md20050334
Shi T, Li X-Q, Wang Z-M, Zheng L, Yu Y-Y, Dai J-J, Shi D-Y. Bioactivity-Guided Screening of Antimicrobial Secondary Metabolites from Antarctic Cultivable Fungus Acrostalagmus luteoalbus CH-6 Combined with Molecular Networking. Marine Drugs. 2022; 20(5):334. https://0-doi-org.brum.beds.ac.uk/10.3390/md20050334
Chicago/Turabian StyleShi, Ting, Xiang-Qian Li, Ze-Min Wang, Li Zheng, Yan-Yan Yu, Jia-Jia Dai, and Da-Yong Shi. 2022. "Bioactivity-Guided Screening of Antimicrobial Secondary Metabolites from Antarctic Cultivable Fungus Acrostalagmus luteoalbus CH-6 Combined with Molecular Networking" Marine Drugs 20, no. 5: 334. https://0-doi-org.brum.beds.ac.uk/10.3390/md20050334